Abstract
Actinic keratoses are hyperkeratotic skin lesions that represent focal abnormal proliferation of epidermal keratinocytes. The major cause of actinic keratoses in otherwise healthy Caucasians appears to be exposure to the sun. Since they are commonly associated with squamous cell carcinoma (SCC) of photodamaged skin, therapy and prevention of these actinic keratoses are important steps to decrease the occurrence of SCC.
New approaches to prevention include new advances in sunscreen that can block ultraviolet-A (UVA) rays more effectively. The major new advance in this regard is the use of microfine zinc oxide in sunscreens.
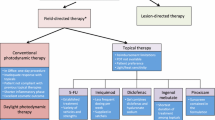
Photodynamic therapy of actinic keratoses can now be done by first applying an aminolevulinic acid (ALA) solution to the site, followed 14–18 hours later by photoactivation using a fluorescent blue light source. ALA is a pro-drug that is not photoactive; it is converted in the actinic keratosis to protoporphyrin disodium, which is the photoactive form of the drug. The complete response rate (complete clearing of actinic keratoses, evaluated individually) was 84% with ALA photodynamic therapy (ALA-PDT).
A topical 3% diclofenac gel (Solaraze™) is US FDA approved to treat actinic keratoses; it has a maximum complete response rate of about 47%. In addition, a topical 5% imiquimod cream (Aldara™) is also being used to treat actinic keratoses (off-label use), with complete clearing response rates of 40–87%.
A 0.5% fluorouracil cream product in a new microsponge delivery system (Carac®) has been approved by the US FDA for treatment of actinic keratoses of the face and scalp. This product is approved for once daily application and completely clears 48% of target actinic keratoses. The marketing of the fluorouracil cream suggests that since only l/40th as much fluorouracil is absorbed systemically, it is theoretically safer for treatment of actinic keratoses and may minimize adverse reactions that could occur in patients with dihydropyrimidine dehydrogenase (DPD) deficiency.
In this review we re-evaluate the status of studies using the older 2–5% fluorouracil (Efudex®) products to treat actinic keratoses as well as do a postmarketing review of adverse reactions with these older, well established products. The limited studies available suggest a complete response rate of 75–86% with 2–5% Efudex®. A postmarketing evaluation of 31 years of adverse drug reaction reports is presented, which demonstrates that the incidence of severe systemic reactions is very low and in the range of 1 in 1 949 288 prescriptions (seven cases in 31 years). A relationship between DPD deficiency and severe systemic toxicity is not suggested by these postmarketing adverse drug reaction data. Furthermore, recent studies in cancer patients suggest that DPD deficiency does not by itself explain severe reactions to systemically administered fluorouracil. A detailed comparison of Carac® and Efudex® is presented.
















Similar content being viewed by others
References
Jeffes EWB, Tang EH. Actinic keratosis: current treatment options. Am J Clin Dermatol 2000; 1(3): 167–79
Kirkham N. Tumors and cysts of the epidermis. In: Elder D, Elenitsas R, Jaworsky C, et al., editors. Lever’s histopathology of the skin. P iladelphia (PA): Raven, 1997: 701–5
Olsen EA, Abernethy L, Kulp-Shorten C, et al. A double blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratosis on the head and neck. J Am Acad Dermatol 1991; 24: 738–43
Black HS, Herd A, Goldberg LH, et al. Effect of low-fat diet on the incidence of actinic keratosis. N Engl J Med 1994; 330: 1272–5
Black HS. Influence of dietary factors on actinically-induced skin cancer. Mutat Res 1998; 422: 185–90
Barta U, Gräfe T, Wollina U. Radiation therapy for extensive actinic keratosis. J Eur Acad Dermatol Venereol 2000; 14: 293–5
Naylor MF, Boyd A, Smith DW, et al. High sun protection factor sunscreens in the suppression of actinic neoplasia. Arch Dermatol 1995; 131: 170–5
Mitchnick MA, Fairhurst D, Pinnell SR. Microfine zinc oxide (Z-Cote) as a photostable UVA/UVB sunblock agent. J Am Acad Dermatol 1999; 40(1): 85–90
Aldara imiquimod cream 5%: company product report. St. Paul (MN): 3M Pharmaceuticals, 2001: 55133–3275
Levulan Kerastick: FDA package insert. Wilmington (MA): DUSA Pharmaceuticals Inc., 2001: 01887
Solaraze diclofenac sodium gel 3%: company product report. Malvern (PA): Bioglan Pharma Inc., 2001: 19355
Efudex® fluorouracil cream and solution 5%: company product report. Costa Mesa (CA): ICN Pharmaceuticals Inc., 2001: 92626
Pearlman DL. Weekly pulse dosing: effective and comfortable topical fluorouracil treatment of multiple facial actinic keratoses. J Am Acad Dermatol 1991; 25: 665–7
Carac® fluorouracil cream 0.5%: FDA package insert. Berwyn (PA): Dermik Laboratories Inc., 2001: 19312
Z-Cote microfine zinc oxide: company educational pamphlet. A sunscreen primer with emphasis on microfine zinc oxide as a transparent full-spectrum sunblock. Wainscott (NY): SunSmart Inc., 2001: 11975
Z-Cote microfine zinc oxide: company product report. Bethlehem (PA): Applied Therapeutics, distributed by NPR Healthcare, 2001; 18015
Solbar Zinc microfine zinc oxide: company product report. Glendale (CA): Person & Covey Inc., 2001: 91221-5018
Stockfleth E, Meyer T, Bennighoff B, et al. Successful treatment of actinic keratosis with imiquimod cream 5%: a report of six cases. Br J Dermatol 2001; 144: 1050–3
Jeffes EWB, McCullough JL, Weinstein GD, et al. Photodynamic therapy of actinic keratoses with topical aminolevulinic acid hydrochloride and fluorescent blue light. J Am Acad Dermatol 2001; 45: 96–104
Weiss J, Furst K, Connolly M, et al. Actinic keratosis may be treated effectively and safely with once-daily 5-fluorouracil 0.5% topical cream [poster]. 59th Annual Meeting of American Academy of Dermatology; 2001 Mar 2–7; Washington, DC
Lawrence N, Cox SE, Cockerell CJ, et al. A comparison of the efficacy and safety of Jessner’s solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Arch Dermatol 1995; 131: 176–81
Szeimies RM, Karrer S, Radakovic-Fijan, et al. Photodynamic therapy using topical methyl-5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol 2002; 47: 258–62
Kurwa HA, Yong-Gee SA, Seed PT, et al. A randomized paired comparison of photodynamic therapy and topical 5-fluorouracil in the treatment of actinic keratoses. J Am Acad Dermatol 1999; 41 (3 Pt 1): 414–8
Rivers JK, McLean DI. An open label study to assess the efficacy and safety of topical 3% diclofenac in a 2.5% hyaluronic acid gel for the treatment of actinic keratoses. Arch Dermatol 1997; 133: 1239–42
Carac® fluorouracil cream 0.5%: company product report. Bridgewater (NJ): Aventis Pharma, 2001: 08807-2854
Efudex® fluorouracil cream and solution 5%: FDA package insert. Costa Mesa (CA): ICN Pharmaceuticals Inc., 2001: 92626
Epstein E. Does intermittent “pulse” topical 5-fluorouracil therapy allow destruction of actinic keratoses without significant inflammation? J Am Acad Dermatol 1998; 38: 77–80
Jorizzo J, Weiss J, Furst K, et al. Safety of once-daily 0.5% 5-fluorouracil topical cream in the treatment of actinic keratosis [poster]. 59th Annual Meeting of American Academy of Dermatology; 2001 Mar 2–7; Washington, DC
Loven K, Stein L, Furst K, et al. Evaluation of the efficacy and tolerability of 0.5% fluorouracil cream and 5% fluorouracil cream applied to each side of the face in patients with actinic keratosis. Clin Ther 2002; 24(6): 990–1000
Levy S, Furst K, Chern W. Pharmacokinetics of topically applied fluorouracil in patients with actinic keratosis [poster]. 59th Annual Meeting of American Academy of Dermatology; 2001 Mar 2–7; Washington, DC
Johnson MR, Hageboutros A, Wang K, et al. Toxicity attributed to topical fluorouracil (5-FU) in a dihydropyrimidine dehydrogenase deficient (DPD) patient. Clin Cancer Res 1999; 6: 2006–11
Johnson MR, Diasio RB. Importance of dihydropyrimidine dehydrogenase (DPD) deficiency in patients exhibiting toxicity following treatment with fluorouracil. Adv Enzyme Regul 2001; 41: 151–7
Wei X, McLeod HL, McMurrough J, et al. Molecular basis of the human dihydropyrimidine dehydrogenase deficiency and 5-fluorouracil toxicity. J Clin Invest 1996; 98(3): 610–5
Yamaguchi K, Arai Y, Kanda Y, et al. Germline mutation of dihydropyrimidine dehydrogenase among a Japanese population in relation to toxicity to fluorouracil. Jpn J Cancer Res 2001; 92(3): 337–43
Acknowledgements
Dr Jeffes is a consultant for ICM Pharm and has done contract work for them. ICN provided funding to support the production of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeffes, E.W.B., Chen, J.T. New Approaches to the Treatment of Actinic Keratosis. Am J Cancer 2, 151–168 (2003). https://doi.org/10.2165/00024669-200302030-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024669-200302030-00001