Abstract
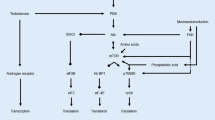
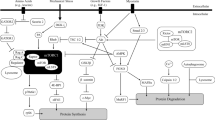
Recent advances in molecular biology have elucidated some of the mechanisms that regulate skeletal muscle growth. Logically, muscle physiologists have applied these innovations to the study of resistance exercise (RE), as RE represents the most potent natural stimulus for growth in adult skeletal muscle. However, as this molecular-based line of research progresses to investigations in humans, scientists must appreciate the fundamental principles of RE to effectively design such experiments. Therefore, we present herein an updated paradigm of RE biology that integrates fundamental RE principles with the current knowledge of muscle cellular and molecular signalling. RE invokes a sequential cascade consisting of: (i) muscle activation; (ii) signalling events arising from mechanical deformation of muscle fibres, hormones, and immune/inflammatory responses; (iii) protein synthesis due to increased transcription and translation; and (iv) muscle fibre hypertrophy. In this paradigm, RE is considered an ‘upstream’ signal that determines specific downstream events. Therefore, manipulation of the acute RE programme variables (i.e. exercise choice, load, volume, rest period lengths, and exercise order) alters the unique ‘fingerprint’ of the RE stimulus and subsequently modifies the downstream cellular and molecular responses.




Similar content being viewed by others
References
Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol 1965; 28: 560–80
Linnamo V, Newton RU, Häkkinen K, et al. Neuromuscular responses to explosive and heavy resistance loading. J Electromyogr Kinesiol 2000; 10 (6): 417–24
Pincivero DM, Gandhi V, Timmons MK, et al. Quadriceps femoris electromyogram during concentric, isometric and eccentric phases of fatiguing dynamic knee extensions. J Biomech 2006; 39 (2): 246–54
Parkington JD, Siebert AP, Le Brasseur NK, et al. Differential activation of mTOR signaling by contractile activity in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2003; 285 (5): R1086–90
Mc Call GE, Byrnes WC, Dickinson A, et al. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol 1996; 81 (5): 2004–12
Trappe SW, Trappe TA, Lee GA, et al. Comparison of a space shuttle flight (STS−78) and bed rest on human muscle function. J Appl Physiol 2001; 91 (1): 57–64
Hornberger TA, Stuppard R, Conley KE, et al. Mechanical stimuli regulate rapamycin—sensitive signalling by a phosphoinositide 3−kinase—, protein kinase B— and growth factor—independent mechanism. Biochem J 2004; 380 (Pt 3): 795–804
Atherton PJ, Babraj J, Smith K, et al. Selective activation of AMPK—PGC−1alpha or PKB—TSC2−mTOR signaling can explain specific adaptive responses to endurance or resistance training—like electrical muscle stimulation. FASEB J 2005; 19 (7): 786–8
Bodine SC, Stitt TN, Gonzalez M, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 2001; 3 (11): 1014–9
Hornberger TA, Chu WK, Mak YW, et al. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci U S A 2006; 103 (12): 4741–6
Park JB, Kim JH, Kim Y, et al. Cardiac phospholipase D2 localizes to sarcolemmal membranes and is inhibited by alpha—actinin in an ADP—ribosylation factor—reversible manner. J Biol Chem 2000; 275 (28): 21295–301
Terada N, Patel HR, Takase K, et al. Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins. Proc Natl Acad Sci U S A 1994; 91 (24): 11477–81
Kimball SR, Farrell PA, Jefferson LS. Invited review: role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol 2002; 93 (3): 1168–80
Sartorelli V, Fulco M. Molecular and cellular determinants of skeletal muscle atrophy and hypertrophy. Sci STKE 2004; 2004 (244): re11
Winder WW, Taylor EB, Thomson DM. Role of AMP—activated protein kinase in the molecular adaptation to endurance exercise. Med Sci Sports Exerc 2006; 38 (11): 1945–9
Bolster DR, Crozier SJ, Kimball SR, et al. AMP—activated protein kinase suppresses protein synthesis in rat skeletal muscle through down—regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 2002; 277 (27): 23977–80
Kimball SR. Interaction between the AMP—activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc 2006; 38 (11): 1958–64
Dreyer HC, Fujita S, Cadenas JG, et al. Resistance exercise increases AMPK activity and reduces 4E—BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 2006; 576 (Pt 2): 613–24
Long YC, Widegren U, Zierath JR. Exercise—induced mitogen activated protein kinase signalling in skeletal muscle. Proc Nutr Soc 2004; 63 (2): 227–32
Hawley JA, Zierath JR. Integration of metabolic and mitogenic signal transduction in skeletal muscle. Exerc Sport Sci Rev 2004; 32 (1): 4–8
Coffey VG, Zhong Z, Shield A, et al. Early signaling responses to divergent exercise stimuli in skeletal muscle from well trained humans. FASEB J 2006; 20 (1): 190–2
Kraemer WJ, Marchitelli L, Gordon SE, et al. Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol 1990; 69 (4): 1442–50
Hansen S, Kvorning T, Kjaer M, et al. The effect of short—term strength training on human skeletal muscle: the importance of physiologically elevated hormone levels. Scand J Med Sci Sports 2001; 11 (6): 347–54
Wilkinson SB, Tarnopolsky MA, Grant EJ, et al. Hypertrophy with unilateral resistance exercise occurs without increases in endogenous anabolic hormone concentration. Eur J Appl Physiol 2006; 98 (6): 546–55
Baumann G. Growth hormone heterogeneity: genes, isohormones, variants, and binding proteins. Endocr Rev 1991; 12 (4): 424–49
Hymer WC, Kraemer WJ, Nindl BC, et al. Characteristics of circulating growth hormone in women after acute heavy resistance exercise. Am J Physiol Endocrinol Metab 2001; 281 (4): E878–87
Nindl BC, Kraemer WJ, Marx JO, et al. Growth hormone molecular heterogeneity and exercise. Exerc Sport Sci Rev 2003; 31 (4): 161–6
Nindl BC. Exercise modulation of growth hormone isoforms: current knowledge and future directions for the exercise endocrinologist. Br J Sports Med 2007; 41 (6): 346–8
Kraemer WJ, Nindl BC, Marx JO, et al. Chronic resistance training in women potentiates growth hormone in vivo bioactivity: characterization of molecular mass variants. Am J Physiol Endocrinol Metab 2006; 291 (6): E1177–87
Piwien-Pilipuk G, Huo JS, Schwartz J. Growth hormone signal transduction. J Pediatr Endocrinol Metab 2002; 15 (6): 771–86
Bush JA, Kimball SR, O’Connor PM, et al. Translational control of protein synthesis in muscle and liver of growth hormone—treated pigs. Endocrinology 2003; 144 (4): 1273–83
Bamman MM, Shipp JR, Jiang J, et al. Mechanical load in creases muscle IGF—I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab 2001; 280 (3): E383–90
Nindl BC, Kraemer WJ, Marx JO, et al. Overnight responses of the circulating IGF—I system after acute, heavy—resistance exercise. J Appl Physiol 2001; 90 (4): 1319–26
Rommel C, Bodine SC, Clarke BA, et al. Mediation of IGF−1−induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 2001; 3 (11): 1009–13
Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol 2007; 103 (1): 378–87
Hawke TJ. Muscle stem cells and exercise training. Exerc Sport Sci Rev 2005; 33 (2): 63–8
Le Roith D, Bondy C, Yakar S, et al. The somatomedin hypothesis: 2001. Endocr Rev 2001; 22 (1): 53–74
Kraemer WJ, Aguilera BA, Terada M, et al. Responses of IGF—I to endogenous increases in growth hormone after heavy—resistance exercise. J Appl Physiol 1995; 79 (4): 1310–5
Hameed M, Lange KH, Andersen JL, et al. The effect of recombinant human growth hormone and resistance training on IGF—I mRNA expression in the muscles of elderly men. J Physiol 2004; 555 (Pt 1): 231–40
Kraemer WJ, Häkkinen K, Newton RU, et al. Acute hormonal responses to heavy resistance exercise in younger and older men. Eur J Appl Physiol Occup Physiol 1998; 77 (3): 206–11
Chandler RM, Byrne HK, Patterson JG, et al. Dietary supplements affect the anabolic hormones after weight—training exercise. J Appl Physiol 1994; 76 (2): 839–45
Bloomer RJ, Sforzo GA, Keller BA. Effects of meal form and composition on plasma testosterone, cortisol, and insulin following resistance exercise. Int J Sport Nutr Exerc Metab 2000; 10 (4): 415–24
Kraemer WJ, Gordon SE, Fleck SJ, et al. Endogenous anabolic hormonal and growth factor responses to heavy resistance exercise in males and females. Int J Sports Med 1991; 12 (2): 228–35
Inoue K, Yamasaki S, Fushiki T, et al. Androgen receptor antagonist suppresses exercise—induced hypertrophy of skeletal muscle. Eur J Appl Physiol Occup Physiol 1994; 69 (1): 88–91
Sinha-Hikim I, Roth SM, Lee MI, et al. Testosterone—induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab 2003; 285 (1): E197–205
Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care 2004; 7 (3): 271–7
Proske U, Allen TJ. Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev 2005; 33 (2): 98–104
Peake J, Nosaka K, Suzuki K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc Immunol Rev 2005; 11: 64–85
Del Aguila LF, Krishnan RK, Ulbrecht JS, et al. Muscle damage impairs insulin stimulation of IRS−1, PI 3−kinase, and Aktkinase in human skeletal muscle. Am J Physiol Endocrinol Metab 2000; 279 (1): E206–12
Kirwan JP, del Aguila LF. Insulin signalling, exercise and cellular integrity. Biochem Soc Trans 2003; 31 (Pt 6): 1281–5
Adams GR, Caiozzo VJ, Haddad F, et al. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol 2002; 283 (4): C1182–95
Alway SE, Gonyea WJ, Davis ME. Muscle fiber formation and fiber hypertrophy during the onset of stretch—overload. Am J Physiol 1990; 259 (1 Pt 1): C92–102
Kraemer WJ. Exercise prescription in weight training: manipulating program variables. Nat Strength Cond Assoc J 1983; 5: 58–9
Toigo M, Boutellier U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur J Appl Physiol 2006; 97 (6): 643–63
Ballor DL, Becque MD, Katch VL. Metabolic responses during hydraulic resistance exercise. Med Sci Sports Exerc 1987; 19 (4): 363–7
Fahey TD, Rolph R, Moungmee P, et al. Serum testosterone, body composition, and strength of young adults. Med Sci Sports 1976; 8 (1): 31–4
Eliasson J, Elfegoun T, Nilsson J, et al. Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab 2006; 291 (6): E1197–205
Garma TM, Kobayashi CA, Haddad F, et al. Similar acute molecular responses to equivalent volumes of isometric, lengthening or shortening mode resistance exercise. J Appl Physiol 2007; 102 (1): 135–43
Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol 1999; 276 (1 Pt 1): C120–7
Bolster DR, Kubica N, Crozier SJ, et al. Immediate response of mammalian target of rapamycin (mTOR)—mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol 2003; 553 (Pt 1): 213–20
Haddad F, Adams GR, Bodell PW, et al. Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J Appl Physiol 2006; 100 (2): 433–41
Dudley GA, Tesch PA, Miller BJ, et al. Importance of eccentric actions in performance adaptations to resistance training. Aviat Space Environ Med 1991; 62 (6): 543–50
Sforzo FA, Touey PR. Manipulating exercise order affects muscular performance during a resistance exercise training session. J Strength Cond Res 1996; 10: 20–4
Komi PV, Kaneko M, Aura O. EMG activity of the leg extensor muscles with special reference to mechanical efficiency in concentric and eccentric exercise. Int J Sports Med 1987; 8 Suppl. 1: 22–9
Gotshalk LA, Loebel CC, Nindl BC, et al. Hormonal responses of multiset versus single—set heavy—resistance exercise protocols. Can J Appl Physiol 1997; 22 (3): 244–55
Campos GE, Luecke TJ, Wendeln HK, et al. Muscular adaptations in response to three different resistance—training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol 2002; 88 (1-2): 50–60
Nader GA, Esser KA. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol 2001; 90 (5): 1936–42
Deschenes MR, Kraemer WJ. Performance and physiologic adaptations to resistance training. Am J Phys Med Rehabil 2002; 81 (11 Suppl.): 3–16
Häkkinen K, Pakarinen A, Alen M, et al. Relationships between training volume, physical performance capacity, and serum hormone concentrations during prolonged training in elite weight lifters. Int J Sports Med 1987; 8 Suppl. 1: 61–5
Willoughby DS, Chilek DR, Schiller DA, et al. The metabolic effects of three different free weight parallel squatting intensities. J Hum Mov Stud 1991; 21: 53–67
Berger RA. Effect of varied weight training programs on strength. Res Q 1962; 33: 168–81
Borst SE, De Hoyos DV, Garzarella L, et al. Effects of resistance training on insulin—like growth factor—I and IGF binding proteins. Med Sci Sports Exerc 2001; 33 (4): 648–53
Sanborn K, Boros R, Hruby J, et al. Short—term performance effects of weight training with multiple sets not to failure vs a single set to failure in women. J Strength Cond Res 2000; 14: 328–31
Stowers T, Mc Millian J, Scala D, et al. The short—term effects of three different strength—power training models. NSCA J 1983; 5: 24–7
Steinberg GR, Watt MJ, Mc Gee SL, et al. Reduced glycogen availability is associated with increased AMPKa2 activity, nuclear AMPKa2 protein abundance, and GLUT4 mRNA expression in contracting human skeletal muscle. Appl Physiol Nutr Metab 2006; 31 (3): 302–12
Churchley EG, Coffey VG, Pedersen DJ, et al. Influence of preexercise muscle glycogen content on transcriptional activity of metabolic and myogenic genes in well—trained humans. J Appl Physiol 2007; 102 (4): 1604–11
Creer A, Gallagher P, Slivka D, et al. Influence of muscle glycogen availability on ERK1/2 and Akt signaling after resistance exercise in human skeletal muscle. J Appl Physiol 2005; 99 (3): 950–6
Wong TS, Booth FW. Protein metabolism in rat tibialis anterior muscle after stimulated chronic eccentric exercise. J Appl Physiol 1990; 69 (5): 1718–24
Wong TS, Booth FW. Protein metabolism in rat gastrocnemius muscle after stimulated chronic concentric exercise. J Appl Physiol 1990; 69 (5): 1709–17
Wong TS, Booth FW. Skeletal muscle enlargement with weightlifting exercise by rats. J Appl Physiol 1988; 65 (2): 950–4
Kraemer WJ, Fleck SJ, Dziados JE, et al. Changes in hormonal concentrations after different heavy—resistance exercise protocols in women. J Appl Physiol 1993; 75 (2): 594–604
Robinson JM, Stone MH, Johnson RL, et al. Effects of different weight training exercise/rest intervals on strength, power, and high intensity exercise endurance. J Strength Cond Res 1995; 9: 216–21
Kraemer WJ, Adams K, Cafarelli E, et al. American College of Sports Medicine position stand: progression models in resistance training for healthy adults. Med Sci Sports Exerc 2002; 34 (2): 364–80
Kraemer WJ, Noble BJ, Clark MJ, et al. Physiologic responses to heavy—resistance exercise with very short rest periods. Int J Sports Med 1987; 8 (4): 247–52
Spreuwenberg LP, Kraemer WJ, Spiering BA, et al. Influence of exercise order in a resistance—training exercise session. J Strength Cond Res 2006; 20 (1): 141–4
Häkkinen K, Komi PV, Alen M. Effect of explosive type strength training on isometric force— and relaxation—time, electromyographic and muscle fibre characteristics of leg extensor muscles. Acta Physiol Scand 1985; 125 (4): 587–600
Augustsson J, Thomee R, Hornstedt P, et al. Effect of preexhaustion exercise on lower—extremity muscle activation during a leg press exercise. J Strength Cond Res 2003; 17 (2): 411–6
Rasmussen BB, Phillips SM. Contractile and nutritional regulation of human muscle growth. Exerc Sport Sci Rev 2003; 31 (3): 127–31
Wolfe RR. Effects of amino acid intake on anabolic processes. Can J Appl Physiol 2001; 26 Suppl.: 220–7
Rennie MJ, Bohe J, Smith K, et al. Branched—chain amino acids as fuels and anabolic signals in human muscle. J Nutr 2006; 136 (1 Suppl.): 264S–8
Blomstrand E, Eliasson J, Karlsson HK, et al. Branched—chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr 2006; 136 (1 Suppl.): 269S–73
Acknowledgments
This work was supported, in part, by graduate student research grants awarded by the National Strength and Conditioning Association and the University of Connecticut. The authors would like to thank Dr Daniel A. Judelson for critically reading the manuscript and providing thoughtful comments, and Dr P. Courtney Gaine for her insightful discussions. The authors have no conflicts of interest directly relevant to the contents of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spiering, B.A., Kraemer, W.J., Anderson, J.M. et al. Resistance Exercise Biology. Sports Med 38, 527–540 (2008). https://doi.org/10.2165/00007256-200838070-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00007256-200838070-00001