Abstract
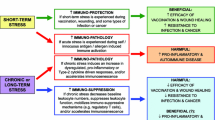
Athletes, military personnel, fire fighters, mountaineers and astronauts may be required to perform in environmental extremes (e.g. heat, cold, high altitude and microgravity). Exercising in hot versus thermoneutral conditions (where core temperature is ≥1°C higher in hot conditions) augments circulating stress hormones, catecholamines and cytokines with associated increases in circulating leukocytes. Studies that have clamped the rise in core temperature during exercise (by exercising in cool water) demonstrate a large contribution of the rise in core temperature in the leukocytosis and cytokinaemia of exercise. However, with the exception of lowered stimulated lymphocyte responses after exercise in the heat, and in exertional heat illness patients (core temperature >40°C), recent laboratory studies show a limited effect of exercise in the heat on neutrophil function, monocyte function, natural killer cell activity and mucosal immunity. Therefore, most of the available evidence does not support the contention that exercising in the heat poses a greater threat to immune function (vs thermoneutral conditions).
From a critical standpoint, due to ethical committee restrictions, most laboratory studies have evoked modest core temperature responses (<39°C). Given that core temperature during exercise in the field often exceeds levels associated with fever and hyperthermia (>39.5°C) field studies may provide an opportunity to determine the effects of severe heat stress on immunity. Field studies may also provide insight into the possible involvement of immune modulation in the aetiology of exertional heat stroke (core temperature >40.6°C) and identify the effects of acclimatisation on neuroendocrine and immune responses to exercise-heat stress. Laboratory studies can provide useful information by, for example, applying the thermal clamp model to examine the involvement of the rise in core temperature in the functional immune modifications associated with prolonged exercise.
Studies investigating the effects of cold, high altitude and microgravity on immunity and infection incidence are often hindered by extraneous stressors (e.g. isolation). Nevertheless, the available evidence does not support the popular belief that short- or long-term cold exposure, with or without exercise, suppresses immunity and increases infection incidence. In fact, controlled laboratory studies indicate immuno-stimulatory effects of cold exposure.
Although some evidence shows that ascent to high altitude increases infection incidence, clear conclusions are difficult to make because of some overlap with the symptoms of acute mountain sickness. Studies have reported suppressed cell-mediated immunity in mountaineers at high altitude and in astronauts after re-entering the normal gravity environment; however, the impact of this finding on resistance to infection remains unclear.








Similar content being viewed by others
References
Brenner IK, Shek PN, Shephard RJ. Infection in athletes. Sports Med 1994; 17: 86–107
Nieman DC, Johanssen LM, Lee JW, et al. Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med Phys Fitness 1990; 30: 316–28
Peters EM, Bateman ED. Ultramarathon running and upper respiratory tract infections: an epidemiological survey. S Afr Med J 1983; 64: 582–4
Kappel M, Tvede N, Galbo H, et al. Evidence that the effect of physical exercise on NK cell activity is mediated by epinephrine. J Appl Physiol 1991; 70: 2530–4
Keast D, Cameron K, Morton AR. Exercise and the immune response. Sports Med 1988; 5: 248–67
Lewicki R, Tchorzewski H, Majewska E, et al. Effect of maximal physical exercise on T-lymphocyte subpopulations and on interleukin 1 (IL 1) and interleukin 2 (IL 2) production in vitro. Int J Sports Med 1988; 9: 114–7
Oshida Y, Yamanouchi K, Hayamizu S, et al. Effect of acute physical exercise on lymphocyte subpopulations in trained and untrained subjects. Int J Sports Med 1988; 9: 137–40
Robson PJ, Blannin AK, Walsh NP, et al. Effects of exercise intensity, duration and recovery on in vitro neutrophil function in male athletes. Int J Sports Med 1999; 20: 128–35
Tomasi TB, Trudeau FB, Czerwinski D, et al. Immune parameters in athletes before and after strenuous exercise. J Clin Immunol 1982; 2: 173–8
Blannin AK, Robson PJ, Walsh NP, et al. The effect of exercising to exhaustion at different intensities on saliva immunoglobulin A, protein and electrolyte secretion. Int J Sports Med 1998; 19: 547–52
Nieman DC, Miller AR, Henson DA, et al. Effects of high- vs moderate-intensity exercise on natural killer cell activity. Med Sci Sports Exerc 1993; 25: 1126–34
Bishop NC, Blannin AK, Walsh NP, et al. Nutritional aspects of immunosuppression in athletes. Sports Med 1999; 28: 151–76
Nieman DC. Exercise immunology: nutritional countermeasures. Can J Appl Physiol 2001; 26: S45–55
Clow A, Hucklebridge F. The impact of psychological stress on immune function in the athletic population. Exerc Immunol Rev 2001; 7: 5–17
Hoffman-Goetz L, Pedersen BK. Exercise and the immune system: a model of the stress response? Immunol Today 1994; 15: 382–7
Shephard RJ. Immune changes induced by exercise in an adverse environment. Can J Physiol Pharmacol 1998; 76: 539–46
Jonsdottir IH. Special feature for the Olympics: effects of exercise on the immune system: neuropeptides and their interaction with exercise and immune function. Immunol Cell Biol 2000; 78: 562–70
Brenner I, Shek PN, Zamecnik J, et al. Stress hormones and the immunological responses to heat and exercise. Int J Sports Med 1998; 19: 130–43
Vale W, Spiess J, Rivier C, et al. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981; 213: 1394–7
Tsigos C, Chrousos GP. Physiology of the hypothalamic-pituitary-adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol Metab Clin North Am 1994; 23: 451–66
Kappel M, Poulsen TD, Hansen MB, et al. Somatostatin attenuates the hyperthermia induced increase in neutrophil concentration. Eur J Appl Physiol 1998; 77: 149–56
Woods J, Lu Q, Ceddia MA, et al. Special feature for the Olympics: effects of exercise on the immune system-exercise-induced modulation of macrophage function. Immunol Cell Biol 2000; 78: 545–53
Madden KS, Felten DL. Experimental basis for neuralimmune interactions. Physiol Rev 1995; 75: 77–106
McCarthy DA, Dale MM. The leucocytosis of exercise: a re-view and model. Sports Med 1988; 6: 333–63
Tonnesen E, Christensen NJ, Brinklov MM. Natural killer cell activity during cortisol and adrenaline infusion in healthy volunteers. Eur J Clin Invest 1987; 17: 497–503
Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab 1999; 10: 359–68
Breuninger LM, Dempsey WL, Uhl J, et al. Hydrocortisone regulation of interleukin-6 protein production by a purified population of human peripheral blood monocytes. Clin Immunol Immunopathol 1993; 69: 205–14
Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 1995; 332: 1351–62
Elenkov IJ, Papanicolaou DA, Wilder RL, et al. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians 1996; 108: 374–81
Waage A, Slupphaug G, Shalaby R. Glucocorticoids inhibit the production of IL6 from monocytes, endothelial cells and fibroblasts. Eur J Immunol 1990; 20: 2439–43
Wu CY, Wang K, McDyer JF, et al. Prostaglandin E2 and dexamethasone inhibit IL-12 receptor expression and IL-12 responsiveness. J Immunol 1998; 161: 2723–30
Morel PA, Oriss TB. Crossregulation between Th1 and Th2 cells. Crit Rev Immunol 1998; 18: 275–303
Ramierz F, Fowell DJ, Puklavec M, et al. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J Immunol 1996; 156: 2406–12
Lindquist S. The heat-shock response. Annu Rev Biochem 1986; 55: 1151–91
Ding XZ, Fernandez-Prada CM, Bhattacharjee AK, et al. Over-expression of hsp-70 inhibits bacterial lipopolysaccharide-induced production of cytokines in human monocyte-derived macrophages. Cytokine 2001; 16: 210–9
Sun D, Chen D, Du B, et al. Heat shock response inhibits NF-kappaB activation and cytokine production in murine Kupffer cells. J Surg Res 2005; 129: 114–21
Moseley PL. Exercise, stress, and the immune conversation. Exerc Sport Sci Rev 2000; 28: 128–32
Asea A. Chaperokine-induced signal transduction pathways. Exerc Immunol Rev 2003; 9: 25–33
Vabulas RM, Ahmad-Nejad P, Ghose S, et al. HSP70 as endogenous stimulus of the toll/interleukin-1 receptor signal pathway. J Biol Chem 2002; 277: 15107–12
Febbraio MA, Ott P, Nielsen HB, et al. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol 2002; 544: 957–62
Fehrenbach E, Niess AM. Role of heat shock proteins in the exercise response. Exerc Immunol Rev 1999; 5: 57–77
Lancaster GI, Moller K, Nielsen B, et al. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones 2004; 9: 276–80
Walsh RC, Koukoulas I, Garnham A, et al. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones 2001; 6: 386–93
Fleshner M, Campisi J, Johnson JD. Can exercise stress facilitate innate immunity? A functional role for stress-induced extracellular Hsp72. Exerc Immunol Rev 2003; 9: 6–24
Fleshner M, Johnson JD. Endogenous extra-cellular heat shock protein 72: releasing signal(s) and function. Int J Hyperthermia 2005; 21: 457–71
Niess AM, Dickhuth HH, Northoff H, et al. Free radicals and oxidative stress in exercise: immunological aspects. Exerc Immunol Rev 1999; 5: 22–56
Bennett IL, Nicastri A. Fever as a mechanism of resistance. Bacteriol Rev 1960; 24: 16–34
Hanson DF. Fever, temperature, and the immune response. Ann N Y Acad Sci 1997; 813: 453–64
Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science 1975; 188: 166–8
Callahan TE, Marins J, Welch WJ, et al. Heat shock attenuates oxidation and accelerates apoptosis in human neutrophils. J Surg Res 1999; 85: 317–22
Fildes J, Fisher S, Sheaff CM, et al. Effects of short heat exposure on human red and white blood cells. J Trauma 1998; 45: 479–84
Frohlich D, Wittmann S, Rothe G, et al. Mild hyperthermia down-regulates receptor-dependent neutrophil function. Anesth Analg 2004; 99: 284–92
Roberts NJ, Steigbigel RT. Hyperthermia and human leukocyte functions: effects on response of lymphocytes to mitogen and antigen and bactericidal capacity of monocytes and neutrophils. Infect Immun 1977; 18: 673–9
Grogan JB, Parks LC, Minaberry D. Polymorphonuclear leukocyte function in cancer patients treated with total body hyperthermia. Cancer 1980; 45: 2611–5
Nakayama J, Nakao T, Mashino T, et al. Kinetics of immunological parameters in patients with malignant melanoma treated with hyperthermic isolated limb perfusion. J Dermatol Sci 1997; 15: 1–8
Park MM, Hornback NB, Endres S, et al. The effect of whole body hyperthermia on the immune cell activity of cancer patients. Lymphokine Res 1990; 9: 213–23
Katschinski DM, Wiedemann GJ, Longo W, et al. Whole body hyperthermia cytokine induction: a review, and unifying hypothesis for myeloprotection in the setting of cytotoxic therapy. Cytokine Growth Factor Rev 1999; 10: 93–7
Robins HI, Cohen JD, Schmitt CL, et al. Phase I clinical trial of carboplatin and 41.8 degrees C whole-body hyperthermia in cancer patients. J Clin Oncol 1993; 11: 1787–94
Robins HI, Longo WL, Lagoni RK, et al. Phase I trial of lonidamine with whole body hyperthermia in advanced cancer. Cancer Res 1988; 48: 6587–92
Robins HI, Dennis WH, Steeves RA, et al. A proposal for the Robins HI, Dennis WH, Steeves RA, et al. A proposal for the chronic leukemia. J Clin Oncol 1984; 2: 1050–6
Ernst E. Sauna: a hobby or for health? J R Soc Med 1989; 82: 639
Ernst E, Pecho E, Wirz P, et al. Regular sauna bathing and the incidence of common colds. Ann Med 1990; 22: 225–7
Cross MC, Radomski MW, VanHelder WP, et al. Endurance exercise with and without a thermal clamp: effects on leukocytes and leukocyte subsets. J Appl Physiol 1996; 81: 822–9
Rhind SG, Gannon GA, Shek PN, et al. Contribution of exertional hyperthermia to sympathoadrenal-mediated lymphocyte subset redistribution. J Appl Physiol 1999; 87: 1178–85
Rhind SG, Gannon GA, Shephard RJ, et al. Cytokine induction during exertional hyperthermia is abolished by core temperature clamping: neuroendocrine regulatory mechanisms. Int J Hyperthermia 2004; 20: 503–16
Pugh LG, Corbett JL, Johnson RH. Rectal temperatures, weight losses, and sweat rates in marathon running. J Appl Physiol 1967; 23: 347–52
Roberts WO. Exercise-associated collapse in endurance events: a classification system. Physician Sportsmed 1989; 17: 49–55
Shephard RJ, Shek PN. Immune dysfunction as a factor in heat illness. Crit Rev Immunol 1999; 19: 285–302
Lim CL, Mackinnon LT. The roles of exercise-induced immune system disturbances in the pathology of heat stroke: the dual pathway model of heat stroke. Sports Med 2006; 36: 39–64
Cohen P, Warren SL. A study of the leukocytosis produced in man by artificial fever. J Clin Invest 1935; 14: 423–33
Downing JF, Taylor MW. The effect of in vivo hyperthermia on selected lymphokines in man. Lymphokine Res 1987; 6: 103–9
Downing JF, Martinez-Valdez H, Elizondo RS, et al. Hyperthermia in humans enhances interferon-gamma synthesis and alters the peripheral lymphocyte population. J Interferon Res 1988; 8: 143–50
Severs Y, Brenner I, Shek PN, et al. Effects of heat and intermittent exercise on leukocyte and sub-population cell counts. Eur J Appl Physiol 1996; 74: 234–45
Kappel M, Stadeager C, Tvede N, et al. Effects of in vivo hyperthermia on natural killer cell activity, in vitro proliferative responses and blood mononuclear cell subpopulations. Clin Exp Immunol 1991; 84: 175–80
Zanker KS, Lange J. Whole body hyperthermia and natural killer cell activity. Lancet 1982; I: 1079–80
Dahn MS, Whitcomb MP, Lange MP, et al. Altered T-lymphocyte subsets in severe sepsis. Am Surg 1988; 54: 450–5
Mackinnon LT. Advances in exercise immunology. Champaign (IL): Human Kinetics, 1999
Bouchama A, al Hussein K, Adra C, et al. Distribution of peripheral blood leukocytes in acute heatstroke. J Appl Physiol 1992; 73: 405–9
Ellis GS, Carlson DE, Hester L, et al. G-CSF, but not corticosterone, mediates circulating neutrophilia induced by febrilerange hyperthermia. J Appl Physiol 2005; 98: 1799–804
Ryan AJ, Flanagan SW, Moseley PL, et al. Acute heat stress protects rats against endotoxin shock. J Appl Physiol 1992; 73: 1517–22
DuBose DA, McCreary J, Sowders L, et al. Relationship between rat heat stress mortality and alterations in reticuloendothelial carbon clearance function. Aviat Space Environ Med 1983; 54: 1090–5
Jiang Q, Cross AS, Singh IS, et al. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun 2000; 68: 1265–70
Marino MW, Dunn A, Grail D, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci U S A 1997; 94: 8093–8
Bouchama A, Knochel JP. Heat stroke. N Engl J Med 2002; 346: 1978–88
Hasday JD, Singh IS. Fever and the heat shock response: distinct, partially overlapping processes. Cell Stress Chaperones 2000; 5: 471–80
D’Oleire F, Schmitt CL, Robins HI, et al. Cytokine induction in humans by 41.8 degrees C whole-body hyperthermia. J Natl Cancer Inst 1993; 85: 833–4
Robins HI, Kutz M, Wiedemann GJ, et al. Cytokine induction by 41.8 degrees C whole body hyperthermia. Cancer Lett 1995; 97: 195–201
Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood 1991; 78: 2791–808
Robins HI, Rushing D, Kutz M, et al. Phase I clinical trial of melphalan and 41.8 degrees C whole-body hyperthermia in cancer patients. J Clin Oncol 1997; 15: 158–64
Nahas GG, Tannieres ML, Lennon JF. Direct measurement of leukocyte motility: effects of pH and temperature. Proc Soc Exp Biol Med 1971; 138: 350–2
Kappel M, Kharazmi A, Nielsen H, et al. Modulation of the counts and functions of neutrophils and monocytes under in vivo hyperthermia conditions. Int J Hyperthermia 1994; 10: 165–73
Bozzetti F, Cozzaglio L, Villa ML, et al. Restorative effect of total parenteral nutrition on natural killer cell activity in malnourished cancer patients. Eur J Cancer 1995; 31A: 2023–7
Puente J, Carvajal T, Parra S, et al. In vitro studies of natural killer cell activity in septic shock patients: response to a challenge with alpha-interferon and interleukin-2. Int J Clin Pharmacol Ther Toxicol 1993; 31: 271–5
Simms HH, Gaither TA, Fries LF, et al. Monokines released during short-term Fc gamma receptor phagocytosis up-regulate polymorphonuclear leukocytes and monocyte-phagocytic function. J Immunol 1991; 147: 265–72
Onozaki K, Matsushima K, Kleinerman ES, et al. Role of interleukin 1 in promoting human monocyte-mediated tumor cytotoxicity. J Immunol 1985; 135: 314–20
Philip R. Cytolysis of tumor necrosis factor (TNF)-resistant tumor targets: differential cytotoxicity of monocytes activated by the interferons, IL-2, and TNF. J Immunol 1988; 140: 1345–9
Northoff H, Berg A, Weinstock C. Similarities and differences of the immune response to exercise and trauma: the IFN-gamma concept. Can J Physiol Pharmacol 1998; 76: 497–504
Ghussen F, Kruger I, Groth W, et al. The role of regional hyperthermic cytostatic perfusion in the treatment of extremity melanoma. Cancer 1988; 61: 654–9
Skene AI, Bulman AS, Williams TR, et al. Hyperthermic isolated perfusion with melphalan in the treatment of advanced malignant melanoma of the lower limb. Br J Surg 1990; 77: 765–7
De Maeyer E, Maeyer-Guignard J. Immuno-modulating properties of interferons. Philos Trans R Soc Lond B Biol Sci 1982; 299: 77–90
Hanson DF, Murphy PA, Silicano R, et al. The effect of temperature on the activation of thymocytes by interleukins I and II. J Immunol 1983; 130: 216–21
Fairchild KD, Viscardi RM, Hester L, et al. Effects of hypothermia and hyperthermia on cytokine production by cultured human mononuclear phagocytes from adults and newborns. J Interferon Cytokine Res 2000; 20: 1049–55
Febbraio MA. Alterations in energy metabolism during exercise and heat stress. Sports Med 2001; 31: 47–59
Galbo H, Houston ME, Christensen NJ, et al. The effect of water temperature on the hormonal response to prolonged swimming. Acta Physiol Scand 1979; 105: 326–37
Galloway SD, Maughan RJ. Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc 1997; 29: 1240–9
Malm C. Exercise immunology: the current state of man and mouse. Sports Med 2004; 34: 555–66
Nieman DC. Current perspective on exercise immunology. Curr Sports Med Rep 2003; 2: 239–42
Mitchell JB, Dugas JP, McFarlin BK, et al. Effect of exercise, heat stress, and hydration on immune cell number and func-tion. Med Sci Sports Exerc 2002; 34: 1941–50
Niess AM, Fehrenbach E, Lehmann R, et al. Impact of elevated ambient temperatures on the acute immune response to intensive endurance exercise. Eur J Appl Physiol 2003; 89: 344–51
Laing S, Blackwell J, Gwynne D, et al. Neutrophil degranulation response to 2h of exercise in a 30°C environment. Aviat Space Environ Med 2005; 76: 1068–73
McFarlin BK, Mitchell JB. Exercise in hot and cold environ-ments: differential effects on leukocyte number and NK cell activity. Aviat Space Environ Med 2003; 74: 1231–6
Brenner IKM, Castellani JW, Gabaree C, et al. Immune changes in humans during cold exposure: effects of prior heating and exercise. J Appl Physiol 1999; 87: 699–710
Pedersen BK, Bruunsgaard H, Klokker M, et al. Exercise-induced immunomodulation: possible roles of neuroendocrine and metabolic factors. Int J Sports Med 1997; 18 Suppl. 1: S2–7
Yamada M, Suzuki K, Kudo S, et al. Raised plasma G-CSF and IL-6 after exercise may play a role in neutrophil mobilization into the circulation. J Appl Physiol 2002; 92: 1789–94
Neville AJ, Sauder DN. Whole body hyperthermia (41–42 degrees C) induces interleukin-1 in vivo. Lymphokine Res 1988; 7: 201–6
Jiang Q, Detolla L, Singh IS, et al. Exposure to febrile temperature upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am J Physiol 1999; 276: R1653–60
Kappel M, Diamant M, Hansen MB, et al. Effects of in vitro hyperthermia on the proliferative response of blood mononuclear cell subsets, and detection of interleukins 1 and 6, tumour necrosis factor-alpha and interferon-gamma. Immunology 1991; 73: 304–8
Pedersen BK. Special feature for the Olympics: effects of exercise on the immune system-exercise and cytokines. Immunol Cell Biol 2000; 78: 532–5
Cross A, Asher L, Seguin M, et al. The importance of a lipopolysaccharide-initiated, cytokine-mediated host defense ichia coli. J Clin Invest 1995; 96: 676–86
Cross AS, Sadoff JC, Kelly N, et al. Pretreatment with recombinant murine tumor necrosis factor alpha/cachectin and murine interleukin 1 alpha protects mice from lethal bacterial infection. J Exp Med 1989; 169: 2021–7
Starkie RL, Hargreaves M, Rolland J, et al. Heat stress, cytokines, and the immune response to exercise. Brain Behav Immun 2005; 19: 404–12
Montain SJ, Latzka WA, Sawka MN. Impact of muscle injury and accompanying inflammatory response on thermoregulation during exercise in the heat. J Appl Physiol 2000; 89: 1123–30
Niess AM, Passek F, Lorenz I, et al. Expression of the antioxidant stress protein heme oxygenase-1 (HO-1) in human leukocytes. Free Rad Bio Med 1999; 26: 184–92
Wilckens T, De Rijk R. Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol Today 1997; 18: 418–24
Laing SJ, Walsh NP, Walters R, et al. The effects of prolonged exercise in a hot environment on lipopolysaccharide (LPS)-stimulated monocyte TNF-a release in trained male cyclists. J Physiol 2003: 555P: PC88
Febbraio MA, Steensberg A, Keller C, et al. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J Physiol 2003; 549: 607–12
Hiscock N, Chan MH, Bisucci T, et al. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J 2004; 18: 992–4
Steensberg A, Febbraio MA, Osada T, et al. Interleukin-6 production in contracting human skeletal muscle is influenced by 633–9 pre-exercise muscle glycogen content. J Physiol 2001; 537: 633–9
Starkie RL, Hargreaves M, Lambert DL, et al. Effect of temperature on muscle metabolism during submaximal exercise in humans. Exp Physiol 1999; 84: 775–84
Febbraio MA, Snow RJ, Hargreaves M, et al. Muscle metabolism during exercise and heat stress in trained men: effect of acclimation. J Appl Physiol 1994; 76: 589–97
Pedersen BK, Febbraio M. Muscle-derived interleukin-6: a possible link between skeletal muscle, adipose tissue, liver, and brain. Brain Behav Immun 2005; 19: 371–6
Febbraio MA, Steensberg A, Starkie RL, et al. Skeletal muscle interleukin-6 and tumor necrosis factor-alpha release in healthy subjects and patients with type 2 diabetes at rest and during exercise. Metabolism 2003; 52: 939–44
Laing SJ, Gwynne D, Blackwell J, et al. Salivary IgA response to prolonged exercise in a hot environment in trained cyclists. Eur J Appl Physiol 2005; 93: 665–71
Muldoon S, Deuster P, Brandom B, et al. Is there a link between malignant hyperthermia and exertional heat illness? Exerc Sport Sci Rev 2004; 32: 174–9
Bouchama A, Parhar RS, el Yazigi A, et al. Endotoxemia and release of tumor necrosis factor and interleukin 1 alpha in acute heatstroke. J Appl Physiol 1991; 70: 2640–4
Hammami MM, Bouchama A, al Sedairy S, et al. Concentrations of soluble tumor necrosis factor and interleukin-6 receptors in heatstroke and heator antagonist. Neurosci Res 1996; 24: 159–63tstress. Crit Care Med 1997; 25: 1314–9
Moseley PL, Gapen C, Wallen ES, et al. Thermal stress induces epithelial permeability. Am J Physiol 1994; 267: C425–34
Hall DM, Buettner GR, Matthes RD, et al. Hyperthermia stimulates nitric oxide formation: electron paramagnetic resonance detection of NO-heme in blood. J Appl Physiol 1994; 77: 548–53
Lambert GP. Role of gastrointestinal permeability in exertional heatstroke. Exerc Sport Sci Rev 2004; 32: 185–90
Gathiram P, Wells MT, Brock-Utne JG, et al. Prevention of endotoxaemia by non-absorbable antibiotics in heat stress. J Clin Pathol 1987; 40: 1364–8
Chiu WT, Kao TY, Lin MT. Increased survival in experimental rat heatstroke by continuous perfusion of interleukin-1 receptor antagonist. Neurosci Res 1996; 24: 159–63
Baker B, Gaffin SL, Wells M, et al. Endotoxaemia in racehorses following exertion. J S Afr Vet Assoc 1988; 59: 63–6
Bosenberg AT, Brock-Utne JG, Gaffin SL, et al. Strenuous exercise causes systemic endotoxemia. J Appl Physiol 1988; 65: 106–8
Brock-Utne JG, Gaffin SL, Wells MT, et al. Endotoxaemia in exhausted runners after a long-distance race. S Afr Med J 1988; 73: 533–6
Jeukendrup AE, Vet-Joop K, Sturk A, et al. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin Sci 2000; 98: 47–55
Marshall JC, Christou NV, Meakins JL. Immunomodulation by altered gastrointestinal tract flora: the effects of orally administered, killed Staphylococcus epidermidis, Candida, and Pseudomonas on systemic immune responses. Arch Surg 1988; 123: 1465–9
Ducreux S, Zorzato F, Muller C, et al. Effect of ryanodine receptor mutations on interleukin-6 release and intracellular calcium homeostasis in human myotubes from malignant hyperthermia-susceptible individuals and patients affected by central core disease. J Biol Chem 2004; 279: 43838–46
Hammami MM, Bouchama A, Shail E, et al. Lymphocyte subsets and adhesion molecules expression in heatstroke and heat stress. J Appl Physiol 1998; 84: 1615–21
DuBose DA, Wenger CB, Flinn SD, et al. Distribution and mitogen response of peripheral blood lymphocytes following exertional heat injury. J Appl Physiol 2003; 95: 2381–9
Castellani JW, IK MB, Rhind SG. Cold exposure: human immune responses and intracellular cytokine expression. Med Sci Sports Exerc 2002; 34: 2013–20
Shephard RJ, Shek PN. Cold exposure and immune function. Can J Physiol Pharmacol 1998; 76: 828–36
Ritzel G. Critical evaluation of vitamin C as a prophylactic and therapeutic agent in colds. Helv Med Acta 1961; 28: 63–8
Sabiston BH, Livingstone SD. Investigation of health problems related to Canadian northern military operations. Toronto: Defence and Civil Institute of Environmental Medicine, 1973
Armstrong LE. Performing in extreme environments. Champaign (IL): Human Kinetics, 2000
Jansky L, Pospisilova D, Honzova S, et al. Immune system of cold-exposed and cold-adapted humans. Eur J Appl Physiol 1996; 72: 445–50
Wenisch C, Narzt E, Sessler DI, et al. Mild intraoperative hypothermia reduces production of reactive oxygen intermediates by polymorphonuclear leukocytes. Anesth Analg 1996; 82: 810–6
Beilin B, Shavit Y, Razumovsky J, et al. Effects of mild perioperative hypothermia on cellular immune responses. Anesthesiology 1998; 89: 1133–40
Lackovic V, Borecky L, Vigas M, et al. Activation of NK cells in subjects exposed to mild hyper- or hypothermic load. J Interferon Res 1988; 8: 393–402
Doubt TJ. Physiology of exercise in the cold. Sports Med 1991; 11: 367–81
Timmons BA, Araujo J, Thomas TR. Fat utilization enhanced by exercise in a cold environment. Med Sci Sports Exerc 1985; 17: 673–8
Pitsiladis YP, Maughan RJ. The effects of exercise and diet manipulation on the capacity to perform prolonged exercise in the heat and in the cold in trained humans. J Physiol 1999; 517: 919–30
Housh TJ, Johnson GO, Housh DJ, et al. The effect of exercise at various temperatures on salivary levels of immunoglobulin A. Int J Sports Med 1991; 12: 498–500
Walsh NP, Blannin AK, Clark AM, et al. The effects of highintensity intermittent exercise on saliva IgA, total protein and alpha-amylase. J Sports Sci 1999; 17: 129–34
Walsh NP, Bishop NC, Blackwell J, et al. Salivary IgA response to prolonged exercise in a cold environment in trained cyclists. Med Sci Sports Exerc 2002; 34: 1632–7
Francis JL, Gleeson M, Lugg DJ, et al. Trends in mucosal immunity in Antarctica during six Australian winter expeditions. Immunol Cell Biol 2002; 80: 382–90
Gleeson M, Francis JL, Lugg DJ, et al. One year in Antarctica: mucosal immunity at three Australian stations. Immunol Cell Biol 2000; 78: 616–22
Bosch JA, Ring C, de Geus EJ, et al. Stress and secretory immunity. Int Rev Neurobiol 2002; 52: 213–53
Stone AA, Cox DS, Valdimarsdottir H, et al. Evidence that secretory IgA antibody is associated with daily mood. J Pers Soc Psychol 1987; 52: 988–93
Williams DL, Climie A, Muller HK, et al. Cell-mediated immunity in healthy adults in Antarctica and the sub-Antarctic. J Clin Lab Immunol 1986; 20: 43–9
Muller HK, Lugg DJ, Quinn D. Cell mediated immunity in Antarctic wintering personnel; 1984–1992. Immunol Cell Biol 1995; 73: 316–20
Gard S. Respiratory virus infections other than influenza. Arch Environ Health 1968; 17: 543–6
Shephard RJ, Castellani JW, Shek PN. Immune deficits induced by strenuous exertion under adverse environmental conditions: manifestations and countermeasures. Crit Rev Immunol 1998; 18: 545–68
Clothier JG. Medical aspects of work in Arctic areas. Practitioner 1974; 213: 805–11
Edwards S, Hucklebridge F, Clow A, et al. Components of the diurnal cortisol cycle in relation to upper respiratory symptoms and perceived stress. Psych Med 2003; 65: 320–7
Giesbrecht GG. The respiratory system in a cold environment. Aviat Space Environ Med 1995; 66: 890–902
Halkier-Sorensen L, Menon GK, Elias PM, et al. Cutaneous barrier function after cold exposure in hairless mice: a model to demonstrate how cold interferes with barrier homeostasis among workers in the fish-processing industry. Br J Dermatol 1995; 132: 391–401
Bailey DM, Davies B, Castell LM, et al. Symptoms of infection and acute mountain sickness; associated metabolic sequelae and problems in differential diagnosis. High Alt Med Biol 2003; 4: 319–31
Singh I, Chohan IS, Lal M, et al. Effects of high altitude stay on the incidence of common diseases in man. Int J Biometeorol 1977; 21: 93–122
Basnyat B, Cumbo TA, Edelman R. Infections at high altitude. Clin Infect Dis 2001; 33: 1887–91
Meehan RT. Immune suppression at high altitude. Ann Emerg Med 1987; 16: 974–9
Ehrlich R, Mieszkuc BJ. Effects of space cabin environment on resistance to infection. I. Effect of 18,000-foot altitude on resistance to respiratory infection. J Infect Dis 1962; 110: 278–81
Highman B, Altland PD. A new method for the production of experimental bacterial endocarditis. Proc Soc Exp Biol Med 1950; 75: 573–7
Meehan R, Duncan U, Neale L, et al. Operation Everest II: alterations in the immune system at high altitudes. J Clin Immunol 1988; 8: 397–406
Facco M, Zilli C, Siviero M, et al. Modulation of immune response by the acute and chronic exposure to high altitude. Med Sci Sports Exerc 2005; 37: 768–74
Pyne DV, McDonald WA, Morton DS, et al. Inhibition of interferon, cytokine, and lymphocyte proliferative responses in elite swimmers with altitude exposure. J Interferon Cytokine Res 2000; 20: 411–8
Biselli R, Le Moli S, Matricardi PM, et al. The effects of hypobaric hypoxia on specific B cell responses following immunization in mice and humans. Aviat Space Environ Med 1991; 62: 870–4
Lancaster GI, Khan Q, Drysdale PT, et al. Effect of prolonged exercise and carbohydrate ingestion on type 1 and type 2 T lymphocyte distribution and intracellular cytokine production in humans. J Appl Physiol 2004; 98: 565–71
Steensberg A, Toft AD, Bruunsgaard H, et al. Strenuous exercise decreases the percentage of type 1 T cells in the circulation. J Appl Physiol 2001; 91: 1708–12
Kleessen B, Schroedl W, Stueck M, et al. Microbial and immunological responses relative to high-altitude exposure in mountaineers. Med Sci Sports Exerc 2005; 37: 1313–8
Bailey DM, Davies B. Physiological implications of altitude training for endurance performance at sea level: a review. Br J Sports Med 1997; 31: 183–90
Mazzeo RS. Altitude, exercise and immune function. Exerc Immunol Rev 2005; 11: 6–16
Pedersen BK, Steensberg A. Exercise and hypoxia: effects on leukocytes and interleukin-6-shared mechanisms? Med Sci Sports Exerc 2002; 34: 2004–13
Klokker M, Kjaer M, Secher NH, et al. Natural killer cell response to exercise in humans: effect of hypoxia and epidural anesthesia. J Appl Physiol 1995; 78: 709–16
Chouker A, Demetz F, Martignoni A, et al. Strenuous physical exercise inhibits granulocyte activation induced by high altitude. J Appl Physiol 2005; 98: 640–7
Levine BD, Stray-Gundersen J. ‘Living high-training low’: effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol 1997; 83: 102–12
Tiollier E, Schmitt L, Burnat P, et al. Living high-training low altitude training: effects on mucosal immunity. Eur J Appl Physiol 2005; 94: 298–304
Fox PC, van der Ven PF, Sonies BC, et al. Xerostomia: evaluation of a symptom with increasing significance. J Am Dent Assoc 1985; 110: 519–25
Gleeson M, McDonald WA, Pyne DB, et al. Salivary IgA levels and infection risk in elite swimmers. Med Sci Sports Exerc 1999; 31: 67–73
Hanson LA, Bjorkander J, Qxelius VA. Selective IgA deficiency. In: Chandra RK, editor. Primary and secondary immunodeficiency disorders. Edinburgh: Churchill Livingstone, 1983: 62–4
Isaacs D, Webster AD, Valman HB. Immunoglobulin levels and function in pre-school children with recurrent respiratory infections. Clin Exp Immunol 1984; 58: 335–40
Jauchem JR. Environmental stressors during space flight: potential effects on body temperature. Comp Biochem Physiol A 1988; 91: 425–9
Dinges DF. Sleep in space flight: breath easy—sleep less? Am J Respir Crit Care Med 2001; 164: 337–8
Mallis MM, DeRoshia CW. Circadian rhythms, sleep, and performance in space. Aviat Space Environ Med 2005; 76: B94–107
Drummer C, Hesse C, Baisch F, et al. Water and sodium balances and their relation to body mass changes in microgravity. Eur J Clin Invest 2000; 30: 1066–75
Smith SM, Davis-Street JE, Rice BL, et al. Nutritional status assessment in semiclosed environments: ground-based and space flight studies in humans. J Nutr 2001; 131: 2053–61
Endler NS. The joint effects of person and situation factors on stress in spaceflight. Aviat Space Environ Med 2004; 75: C22–7
Durnova GN, Kaplansky AS, Portugalov VV. Effect of a 22-day space flight on the lymphoid organs of rats. Aviat Space Environ Med 1976; 47: 588–91
Sonnenfeld G. The immune system in space and microgravity. Med Sci Sports Exerc 2002; 34: 2021–7
Taylor GR. Overview of spaceflight immunology studies. J Leukoc Biol 1993; 54: 179–88
Taylor GR. Immune changes during short-duration missions. J Leukoc Biol 1993; 54: 202–8
Taylor GR, Dardano JR. Human cellular immune responsiveness following space flight. Aviat Space Environ Med 1983; 54: S55–9
Taylor GR, Neale LS, Dardano JR. Immunological analyses of U.S. Space Shuttle crewmembers. Aviat Space Environ Med 1986; 57: 213–7
Barger LK, Greenleaf JE, Baldini F, et al. Effects of space missions on the human immune system: a meta-analysis. Sports Med Training Rehab 1995; 5: 293–310
Borchers AT, Keen CL, Gershwin ME. Microgravity and immune responsiveness: implications for space travel. Nutrition 2002; 18: 889–98
Lange RD, Andrews RB, Gibson LA, et al. Hematological measurements in rats flown on Spacelab shuttle, SL-3. Am J Physiol 1987; 252: R216–21
Sams CF, Crucian BE, Clift VL, et al. Development of a whole blood staining device for use during space shuttle flights. Cytometry 1999; 37: 74–80
Macho L, Kvetnansky R, Fickova M, et al. Endocrine responses to space flights. J Gravit Physiol 2001; 8: 117–20
Tipton CM, Greenleaf JE, Jackson CG. Neuroendocrine and immune system responses with spaceflights. Med Sci Sports Exerc 1996; 28: 988–98
Cogoli A. Space flight and the immune system. Vaccine 1993; 11: 496–503
Konstantinova IV. Immune resistance of man in space flights. Acta Astronaut 1991; 23: 123–7
Chapes SK, Morrison DR, Guikema JA, et al. Production and action of cytokines in space. Adv Space Res 1994; 14: 5–9
Manie S, Konstantinova I, Breittmayer JP, et al. Effects of long duration spaceflight on human T lymphocyte and monocyte activity. Aviat Space Environ Med 1991; 62: 1153–8
Talas M, Batkai L, Stoger I, et al. Results of space experiment program ‘Interferon’. I. Production of interferon in vitro by human lymphocytes aboard space laboratory Solyut-6 (‘Interferon I’) and influence of space flight on lymphocyte functions of cosmonauts (‘Interferon III’). Acta Microbiol Hung 1983; 30: 53–61
Gould CL, Lyte M, Williams J, et al. Inhibited interferon-gamma but normal interleukin-3 production from rats flown on the space shuttle. Aviat Space Environ Med 1987; 58: 983–6
Konstantinova IV, Rykova MP, Lesnyak AT, et al. Immune changes during long-duration missions. J Leukoc Biol 1993; 54: 189–201
Meshkov D, Rykova M. The natural cytotoxicity in cosmonauts on board space stations. Acta Astronaut 1995; 36: 719–26
Taylor GR, Janney RP. In vivo testing confirms a blunting of the human cell-mediated immune mechanism during space flight. J Leukoc Biol 1992; 51: 129–32
Gmunder FK, Konstantinova I, Cogoli A, et al. Cellular immunity in cosmonauts during long duration spaceflight on board the orbital MIR station. Aviat Space Environ Med 1994; 65: 419–23
Sonnenfeld G. Extreme environments and the immune system: effects of spaceflight on immune responses. J Allergy Clin Immunol 2001; 107: 19–20
Castellani JW. Symposium: immune function in environmental extremes — an introduction. Med Sci Sports Exerc 2002; 34: 2002–3
Nickerson CA, Ott CM, Mister SJ, et al. Microgravity as a novel environmental signal affecting Salmonella enterica serovar typhimurium virulence. Infect Immun 2000; 68: 3147–52
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walsh, N.P., Whitham, M. Exercising in Environmental Extremes. Sports Med 36, 941–976 (2006). https://doi.org/10.2165/00007256-200636110-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00007256-200636110-00003