Abstract
Aim
Levocarnitine is an important contributor to cellular energy metabolism. This study aims to evaluate the effects of levocarnitine supplementation on body composition, lipid profile and fatigue in elderly subjects with rapid muscle fatigue.
Method
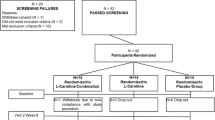
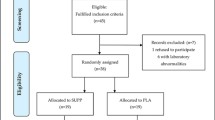
This was a placebo-controlled, randomised, double-blind, two-phase study. Eighty-four elderly subjects with onset of fatigue following slight physical activity were recruited to the study. Prior to randomisation all patients entered a 2-week normalisation phase where they were given an ‘ad libitum’ diet, according to the National Cholesterol Education Program (Step 2). Subjects were asked to record their daily food intake every 2 days. Before the 30-day treatment phase, subjects were randomly assigned to two groups (matched for male/female ratio, age and body mass index). One group received levocarnitine 2g twice daily (n = 42) and the other placebo (n = 42). Efficacy measures included changes in total fat mass, total muscle mass, serum triglyceride, total cholesterol, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), apolipoprotein (apo)A1, and apoB levels. The Wessely and Powell scale was used to evaluate physical and mental fatigue. Subjects were assessed at the beginning and end of the study period.
Results
At the end of the study, compared with placebo, the levocarnitine-treated patients showed significant improvements in the following parameters: total fat mass (−3.1 vs −0.5 kg), total muscle mass (+2.1 vs +0.2 kg), total cholesterol (−1.2 vs +0.1 mmol/L), LDL-C (−1.1 vs −0.2 mmol/L), HDL-C (+0.2 vs +0.01 mmol/ L), triglycerides (−0.3 vs 0.0 mmol/L), apoA1 (−0.2 vs 0.0 g/L), and apoB (−0.3 vs −0.1 g/L). Wessely and Powell scores decreased significantly by 40% (physical fatigue) and 45% (mental fatigue) in subjects taking levocarnitine, compared with 11% and 8%, respectively, in the placebo group (p < 0.001 vs placebo for both parameters). No adverse events were reported in any treatment group.
Conclusion
Administration of levocarnitine to healthy elderly subjects resulted in a reduction of total fat mass, an increase of total muscle mass, and appeared to exert a favourable effect on fatigue and serum lipids.




Similar content being viewed by others
Notes
Use of tradenames is for product identification only and does not imply endorsement.
References
Larsoon L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol 1979; 46: 451–6
Lennmarken C, Bergman T, Larsson J, et al. Skeletal muscle function in man: force, relaxation rate, endurance and contraction time-dependence on sex and age. Clin Physiol 1985; 5: 243–55
Rogers MA, Evans WJ. Changes in skeletal muscle with aging: effects of exercise training. Exerc Sport Sci Rev 1993; 21: 65–102
Wolfson L, Judge J, Whipple R, et al. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol 1995; 50A: 64–7
Baumgartner RN, Waters DL, Gallagher D, et al. Predictors of skeletal muscle mass in elderly man and women. Mech Ageing Dev 1999; 107: 123–36
Kyle UG, Genton L, Hans D, et al. Fat-free and fat mass percentiles of 5225 healthy subjects 15 to 98 years. Nutrition 2001; 17: 534–41
Brenner J. The role of carnitine in cell metabolism. In: De Simone C, Famularo G editors. Carnitine today. Heidelberg: Springer-Verlag, 1997: 1–37
Brenner J. Carnitine metabolism and functions. Physiol Rev 1983; 63: 1420–80
Buechler KF, Lowenstein JM. The involvement of carnitine intermediates in peroxisomal fatty acid oxidation: a study with 2-bromofatty acids. Arch Biochem Biophys 1990; 281: 233–8
Engel AG, Angelini C. Carnitine deficiency of human skeletal muscle with associated lipid storage myopathy: a new syndrome. Science 1973; 179: 899–902
Treem WR, Stanley CA, Finegold DN, et al. Primary carnitine deficiency due to a failure of carnitine transport in kidney, muscle, and fibroblasts. N Engl J Med 1988; 319: 1331–6
Siami G, Clinton ME, Mrak K, et al. Evaluation of the effect of intravenous L-carnitine therapy on function, structure and fatty acid metabolism of skeletal muscle in patients receiving chronic hemodialysis. Nephron 1991; 57: 306–13
Wessely S, Powell R. Fatigue syndromes: a comparison of chronic postviral fatigue with neuromuscular and affective disorders. J Neurol Neurosurg Psych 1989; 52: 940–8
World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997; 277: 925–6
National Cholesterol Education Program. Second Report of the Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). Circulation 1994; 89: 1333–445
Lipid Research Clinics. Population studies data book. Vol. 2. The prevalence study: nutrient intake. Washington, DC. Govt Printing Office, 1982
Centers for Disease Control and Prevention. Prevalence of health care providers asking older adults about their physical activity levels: USA 1998. MMWR Morb Mortal Wkly Rep 2002; 51: 412–4
Rebouche CJ, Seim H. Carnitine metabolism and its regulation in microorganism and mammals. Annu Rev Nutr 1998; 18: 39–61
Plioplys AV, Plioplys S. Amantadine and L-carnitine treatment of chronic fatigue syndrome. Neuropsychobiology 1997; 35: 16–23
Malaguarnera M, Pistone G, Receputo G, et al. Serum carnitine levels in centenarians. Clin Drug Invest 1999; 17: 321–7
Brass EP, Hiatt WR. The role of carnitine and carnitine supplementation during exercise in man and in individuals with special needs. J Am Coll Nutr 1998; 17: 207–15
Villani RG, Gannon J, Self M, et al. L-carnitine supplementation combined with aerobic training does not promote weight loss in moderately obese women. Int J Sport Nutr Exerc Metab 2000; 10: 199–207
Golper TA, Wolfson M, Ahmad S, et al. Multicenter trial of L-carnitine maintenance haemodialysis patients: I. carnitine concentrations and lipid effects. Kidney Int1990; 38: 904–11
Eisaf M, Bairaktari E, Katopodis K, et al. Effect of L-carnitine supplementation on lipid parameters in haemodialysis patients. Am J Nephrol 1998; 18: 416–21
Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med 1997; 337: 1279–84
Dutta C, Hadley EC. The significance of sarcopenia in old age. J Gerontol A Biol Sci Med Sci 1995; 50: 1–4
Malaguarnera M, Maugeri D, Saraceno B, et al. Effects of carnitine on biochemical responses in patients with chronic hepatitis C treated with interferon-α. Clin Drug Invest 2002; 22: 443–8
Andrieu-Abadie N, Jaffrezou JP, Hatem S, et al. L-carnitine prevents doxorubicin-induced apoptosis of cardiac myocytes: role of inhibition of ceramide generation. FASEB J 1999; 13: 1501–10
Acknowledgements
The authors thank Steven Dawber for his expert editorial assistance. No sources of funding were used to assist in the preparation of this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pistone, G., Marino, A.D., Leotta, C. et al. Levocarnitine Administration in Elderly Subjects with Rapid Muscle Fatigue. Drugs Aging 20, 761–767 (2003). https://doi.org/10.2165/00002512-200320100-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002512-200320100-00004