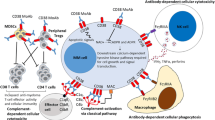
Abstract
In vivo use of monoclonal antibodies (mAbs) has become a mainstay of routine clinical practice in the treatment of various human diseases. A number of molecules can serve as targets, according to the condition being treated. Now entering human clinical trials, CD38 molecule is a particularly attractive target because of its peculiar pattern of expression and its twin role as receptor and ectoenzyme. This review provides a range of analytical perspectives on the current progress in and challenges to anti-CD38 mAb therapy. We present a synopsis of the evidence available on CD38, particularly in myeloma and chronic lymphocytic leukemia (CLL). Our aim is to make the data from basic science helpful and accessible to a diverse clinical audience and, at the same time, to improve its potential for in vivo use. The topics covered include tissue distribution and signal implementation by mAb ligation and the possibility of increasing cell density on target cells by exploiting information about the molecule’s regulation in combination with drugs approved for in vivo use. Also analyzed is the behavior of CD38 as an enzyme: CD38 is a component of a pathway leading to the production of adenosine in the tumor microenvironment, thus inducing local anergy. Consequently, not only might CD38 be a prime target for mAb-mediated therapy, but its functional block may contribute to general improvement in cancer immunotherapy and outcomes.
Similar content being viewed by others
Introduction
The vast majority of today’s therapeutic strategies for cancer treatment involve the targeting of surface molecules expressed by solid tumors or leukemic cells. One such molecule is human CD38, whose essential role in normal cells and in tumor growth has made it useful in the design of effective monoclonal antibody (mAb)-mediated therapeutic options for several forms of human cancer. This review provides a range of analytical perspectives on the current progress in and challenges to anti-CD38 mAb therapy. It also recapitulates the available evidence concerning CD38 expression and dynamics on cell membranes, as well as the signals implemented upon mAb ligation, in light of the molecule’s dual function as receptor and ectoenzyme.
The disease models chosen as candidates for anti-CD38 therapy were myeloma and chronic lymphocytic leukemia (CLL), where CD38 exerts diagnostic and prognostic roles. Despite recent advances in treatment options, both diseases remain incurable.
Special attention is given to evaluating the possibility of increasing surface expression of CD38 (crucial in the case of CLL), with information about its regulation and on the availability of new drugs already approved for in vivo use serving as a basis for potential transferability.
Human CD38 is a surface molecule originally defined as a T-cell activation molecule, although it is no longer considered to be strictly dependent on cell lineage or activation. Mature resting cells and lymphocytes, however, do express limited to nil amounts of surface CD38.
Detailed analysis of the structure and functions of CD38 in humans is aimed at giving clinical scientists an access to background knowledge, usually found only in the context of a basic science (1). The intent is that this set of data may improve the transferability of mAb-mediated therapeutic protocols in clinics.
CD38 AS a Surface Molecule
The protein encoded by CD38 is a single chain type II transmembrane molecule displaying a canonical molecular weight of ≈45 kDa; however, the functional molecule appears as a dimer or a multimer (2–4) or is expressed on the cytoplasmic side of the membrane (5). One function initially attributed to CD38 is the regulation of activation and proliferation of human T lymphocytes (6,7). CD38 ligation by agonistic mAbs induces rapid Ca2+ fluxes and triggers the phosphorylation of a cascade of intracellular substrates, leading to activation of the NF-κB complex (8,9). Protracted effects include initiation of genetic programs causing cytokine secretion and proliferation of T lymphocytes (10). CD31 (also known as platelet endothelial cell adhesion molecule-1 [PECAM-1]) is a CD38 nonsubstrate ligand that can start the signaling cascade and that recapitulates the biological events observed in vitro using surrogate agonistic mAbs (11,12).
The transition from monomers to dimers (or multimers) modulates the functions of the molecule (13). CD38 displays preferential localizations in microdomains of the plasma membrane, in close contact with the BCR complex and with other molecules regulating signaling, homing and adhesion (14).
CD38 in Myeloma
The first question regarding use of CD38 as a model target concerns its distribution in normal and pathological samples. Within the B-cell compartment, CD38 is expressed at high levels only by committed progenitor bone marrow (early BM cells are CD38−) and by B lymphocytes in germinal centers, by terminally differentiated plasma cells and in activated tonsils. Instead, mature virgin and memory B lymphocytes express low levels of the molecule. CD38 also is found in soluble form in normal and pathological fluids (15) and in exosomes, which are membrane vesicles secreted by B cells and likely a component of an intercellular communication network (16).
Clinical routine confirms that the cells of the myeloma clone do express CD38 in the overwhelming majority of patients, although at varying surface densities. A similar analysis has been conducted on a vast panel of human cell lines derived from myeloma and plasma cell leukemia patients. These cell lines are usually used for in vitro and animal in vivo tests to confirm the validity of the working hypothesis (Table 1).
CD38 in Chronic Lymphocytic Leukemia
CLL is a disease characterized by a dynamic balance between cells circulating in the blood and cells located in permissive niches in lymphoid organs. The former are primarily mature-appearing small lymphocytes resistant to apoptosis, whereas the latter are composed of cells that undergo either proliferation or apoptosis according to the microenvironment. The heterogeneous clinical outcome of CLL patients is determined, at least in part, by signals coming from the different environments. Immunophenotypic and genetic analyses of several neoplastic clones led to the identification of a number of molecular markers, some of which have been adopted in clinics to predict disease outcome. Cell surface CD38 is one of these. Association between CD38 expression by CLL cells and a more aggressive clinical behavior was first reported in 1999 (17) and later confirmed by several studies (18). It is now generally accepted that CD38+ patients will have a shorter progression-free interval, require earlier and more frequent treatments, and ultimately die sooner. It remains undeniable that CD38 marks the majority of clinically more aggressive leukemic clones requiring treatment (19).
Interaction between Specific mAbs and Surface CD38
The events taking place between a mAb and its target are of key relevance for in vivo use. The results obtained in normal and tumoral cells show that CD38 endocytosis is a reproducible event which follows CD38 ligation with specific mAbs and involves significant fractions of the entire amount of surface CD38 molecules. It is independent of any signal transduction implemented by mAb ligation, as can be inferred from the observations that both agonistic and nonagonistic mAbs are effective, and that the dynamic of internalization is much slower than that of cellular signaling (20). These results were obtained using intact mAb molecules (Figure 1). The results obtained in myeloma cells in terms of binding also are reproducible in CLL cells.
Confocal microscopy analysis of CD38 internalization. DL06 myeloma cells were incubated with saturating amounts of FITC-labeled agonistic IB4 mAb for 30 min at 4°C. After rinsing in RPMI-1640 medium (Sigma, Milano, Italy) with 5% fetal calf serum (Euroclone, Milano, Italy), affinity-purified goat anti-mouse IgG2a (SouthernBiotech, Birmingham, AL, USA) was added for 10 min at 4°C. Cells were successively incubated at 37°C for selected times. DL06 were then fixed at 4°C for 20 min in PBS containing 4% formalin and rinsed in PBS. The samples were analyzed with an Olympus FV300 laser scanning confocal microscope equipped with a Blue Argon (488 nm) laser and FluoView 300 software (Olympus Biosystems, Hamburg, Germany).
Antibody-mediated therapy may rely upon human or murine antibodies: studies with F(ab′)2 or Fab fragments established that the signals implemented by ligation are reduced or completely abolished.
CD38 As a Receptor
An initial function attributed to CD38 was the regulation of activation of human T lymphocytes, as inferred by the use of agonistic specific mAbs. Early functional studies to identify potential counterreceptors were pursued by monitoring the effects induced by the engagement of different domains of the target by a panel of specific mAbs. The effects induced by ligation with mAb would mimic those elicited by a ligand still unknown at the time. The identification of a first putative ligand come from the observation that human T lymphocytes tend to adhere to endothelial cells (21). Different set of experiments blocking this adhesion concluded that CD31 was crucial to leukocyte adhesion and transmigration (22) and, consequently, it was identified as a nonsubstrate ligand for CD38. It was later demonstrated that CD38/CD31 interactions trigger the same signaling cascade and recapitulate the biological events observed using agonistic mAbs (12,23). The CD38/CD31 cross-talk has been analyzed extensively in a number of different environments ranging from T to B, natural killer (NK), and myeloid cells, in normal and pathological conditions (24). CD38-mediated signals are regulated at distinct levels. The first level concerns the ultrastructural organization of the molecule, which exists both in monomeric, dimeric (or multimeric) type I forms (2–4). A flip-flop mechanism of membrane positioning has been proposed recently, with a type III form of CD38 displaying its catalytic site in the cytoplasm (5). The second level is based on the dynamic localization of CD38 in lipid microdomains within the plasma membrane. Lateral associations with other proteins, which vary according to the cell lineage, determine a third level of control. Lipid raft localization and association with professional signaling complexes are prerequisites for signals mediated through CD38 (25).
A determining factor in supporting the validity of the approach adopted is whether mAb ligation of the CD38 target molecule may implement signals, eventually impairing or disturbing the expected therapeutic effects. For these reasons, cells were exposed to agonistic mAb and the effects tested in terms of changes in transcriptome and in mi-croRNA (miRNA) expression.
Signals Implemented by CD38 Ligation on Target Myeloma Cells
This has been tested using agonistic mAbs on DL06, a continuous cell line secreting IgAλ, and derived from a patient with aggressive myeloma. The results of the microarray analysis are summarized in Figure 2. The analysis of four independent biological replicates of DL06 cells incubated for 2 h/24 h with anti-CD38 mAb and compared with controls revealed no significant modulation of expression across the entire genome (both protein coding and long noncoding RNAs were analyzed). None of the 14 probes differentially expressed by CD38 treatment in at least one of the analyzed biological replicates has an average log2 ratio ≥1 or ≤-1, nor does any of them show a consistent modulation pattern at 2 h or at 24 h.
Comparative microarray analysis of the DL06 cells after CD38 ligation (2 h versus 24 h). RNA was obtained from the DL06 cells incubated at 37°C with anti-CD38 (clone IB4) mAb for 2 h and 24 h. An irrelevant isotype-matched anti-TCRvβ3 mAb was used as control for the same interval times. Four independent experimental settings were analyzed using the two-color comparative hybridization procedure in three cases and the one color protocol in one. mRNA amplification and labeling was followed by hybridization on 8×60K Human Whole Genome Oligo Microarrays (Agilent Technologies, Santa Clara, CA, USA) with technical replication (details in (68)). Images were analyzed using Feature Extraction software v10.5 (Agilent Technologies) and raw data processed within R statistical environment, using the limma library (https://doi.org/www.bioconductor.org/packages/2.11/bioc/html/limma.html). The raw intensity values were background-corrected with the normexp method and normalized with the quantile method, in the case of one-color hybridization. Two-color hybridization arrays were first subjected to Loess intraarray normalization and then to quantile interarray normalization. For each treatment time, technical replicates were combined and the empirical Bayes method was applied to retrieve any modulated probe in treated versus control comparisons (Benjamini-Hochberg corrected p value <0.01). Each point refers to 1 of the 14 transcripts with adjusted p value ≤0.01 in at least one of the experimental samples analyzed. Red represents upregulated expression and green downregulated expression.
We also tested the hypothesis that CD38 signals may operate on a myeloma background by modulating miRNAs. This hypothesis has been confirmed and Figure 3 summarizes the panel of miRNAs up- and downmodulated when DL06 cells are exposed to agonistic anti-CD38 mAb (corrected p value <0.05).
Heat map representation of miRNAs modulated in human DL06 myeloma line by CD38-mediated signals. Total RNA was isolated from DL06 line treated with anti-CD38 (clone IB4) mAb for 0, 2, 6 and 24 h at 4°C. An irrelevant isotype-matched anti-TCRvβ3 mAb was used as control for the same interval times. Two biological replicates for 0, 24 h and control samples were hybridized. miRNA expression was investigated using the Agilent Human miRNA microarray (G4470B, Agilent Technologies), as described (69). By GeneSpring GX 12 software (Agilent Technologies), data transformation was applied to set all the negative raw values at 1.0, followed by a quantile normalization and a log2 transformation. miRNA expression was referred to time 0 h of each biological series. Filters on gene expression were used to keep only the miRNAs that were detected in at least one sample. miRNAs significantly modulated in the DL06 model after 24-h treatment with agonistic anti-CD38 mAb were identified using unpaired t test (corrected p value <0.05). Treatment was included in cluster analysis. The expression values of the genes represented on the heat map correspond to the values normalized on time 0 sample of the same biological series. Red represents upregulated expression and green downregulated expression.
Among the miRNAs downregulated by CD38 ligation, miR-193b functions as a tumor suppressor miRNA in non-small cell lung carcinoma, breast cancer, prostate carcinoma, melanoma, hepatocellular carcinoma and other solid tumors (26–30). In hematologic malignancies, it is a regulator of c-Kit expression in acute myeloid leukemia (31). miR-193b is downregulated in CD38+ myelom as compared with normal CD38+ cells (32). miR-33a is downregulated in colorectal cancer and targets the oncogene Pim-1. miR-33a controls the cycle of normal cells through the modulation of CDK6 and CCND1, with subsequent cell arrest (33). miR-33a also is involved in cholesterol metabolism (34). The overexpression of miR-340 correlates with reduced migration and invasion of breast cancer cells (35).
Signals Implemented by CD38 Ligation on Target CLL Cells
Ligation of CD38 by means of agonistic mAbs or by the CD31 ligand induces proliferation and immunoblast differentiation of CLL cells. More recently, the genetic signature that follows long-term in vitro interactions between CD38+ CLL cells and CD31+ cells has been defined in detail. The emerging profile confirms that the CD31/CD38 axis (surrogated by means of agonistic mAbs) activates genetic programs relevant for proliferative responses. It also indicates that this axis contributes to the processes mediating migration and homing (36). Blocking this network and related signals may provide a simple approach to interfering with the localization of leukemic cells to growth-permissive sites (19).
CD38 As an Inducible Target
The number of surface CD38 target molecules is high on myeloma cells, whereas it is significantly lower in CLL cells. We asked whether the molecule levels could be safely incremented in both cell types.
The promoter of the CD38 gene is highly sensitive to the action of retinoic acid (RA), even at very low concentrations and far below those accepted for in vivo treatment. All-trans retinoic acid (ATRA), an active metabolite of vitamin A, is also the most common drug used for clinical applications. Retinoids influence the regulation of CD38 by binding a family of receptors (RAR), which interacts with retinoic acid response elements (RARE). These elements also have been detected in intron 1 of the promoter of the CD38 gene (37,38). The ATRA/RAR/RARE binding cascade is the early phase of a multistep process (phase 1), which includes a delayed phase mediated through response elements in the 5′-upstream region of CD38 (phase 2). This second step requires de novo synthesis of protein kinase Cδ (PKCδ), which likely acts on a sequence of elements for c/Enhancer Binding Protein β (c/EBPβ), a noncanonical ATRA-responsive element (39).
ATRA activates both phase 1 and phase 2. Sugawara et al. recently showed that new synthetic derivatives of retinoids (for example, tamibarotene, Am80) may contribute to the regulation of CD38 expression, but their effects only involve the early phase response (40). The interactions between retinoids and CD38 response are sketched out in Figure 4.
These observations suggest that tamibarotene may increase the expression of surface CD38, with effects limited to the period adopted for therapy. Consequently, Am80 (and likely other synthetic retinoids) may serve in designing strategies to increase surface CD38, without the side effects associated with ATRA.
Myeloma
The effects elicited by ATRA and tamibarotene were evaluated on CD38 expressed by myeloma cells (freshly isolated from patients) and by myeloma lines (DL06, U266, LP-1, JJN3, OPM-2 and IM9). 24 h treatments with different doses of ATRA or tamibarotene were followed by a modest in vitro increase of surface CD38, in the range of 1.5-fold, although this was in cells already expressing very high levels of the molecule (Figures 5A, C).
CD38 expression on fresh myelomas (A) and human myeloma cell lines (B) after 24 h exposure to ATRA. CD38 expression on fresh myelomas (C) and human myeloma cell lines (D) after 24 h exposure to tamibarotene. Cells were cultured at 37°C with retinoid for 24 h. Cells were then incubated at 4°C for 20 min with the appropriate conjugated antibodies panel. Flow cytometric analyses were performed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA, USA), using a WinMDI 2.9 software.
The effects induced by ATRA or tamibarotene treatments in the myeloma lines expressing different levels of the molecule were marked, ranging from 1.3-and 6.1-fold (Figures 5B, D).
Chronic Lymphocytic Leukemia
The same experiments were performed on CD38+ and CD38− CLL samples. Because CD38+ CLL cells characterize patients with poor clinical outcome, they are potential targets for in vivo treatment. The results obtained using CD38+ samples confirm that ATRA induces a marked increase in the expression of the molecule (as assessed in terms of relative mean fluorenscence intensity [rMFI]), whose surface levels are generally low (Figure 6A).
CD38 expression in ATRA-treated CD19+/CD5+ CLL cells. PBMC isolated from CLL patients (CD38+ CLL, n = 6; CD38− CLL, n = 9) and cultured (24 h at the indicated ATRA concentrations) were stained with anti-CD19, CD5 and CD38 mAbs, all locally produced. CD38 expression was analyzed using FACSDiva software (Becton Dickinson). Representative CD38 expression in CD38+ (A) and CD38− (B) CLL cells from patients treated with ATRA, by evaluating rMFI.
The results obtained with the CD38− CLL cells were quite surprising: indeed, these leukemic cells proved unresponsive to treatment with retinoids (Figure 6B). Lack of response by these cells does not pose an obstacle for the design of therapeutic interventions, for which only CD38+ CLL patients are candidates. In any case, this is a finding of interest in the analysis of the molecule’s regulation, and warrants further investigation in light of the correlation with poly (ADP-ribose) polymerases (PARPs) (41). Methylation on the RARE region of the CD38 promoter has been excluded (S Oliviero, personal communication).
CD38 As an Ectoenzyme
CD38 is a multifunctional enzyme that catalyzes the synthesis of cyclic adenosine diphosphate ribose (cADPR) from nicotinamide adenine dinucleotide (NAD+) and also mediates the hydrolysis of cADPR to ADPR (42–45). In acidic conditions, CD38 catalyzes the generation of nicotinic acid adenine dinucleotide phosphate (NAADP) from nicotinamide adenine dinucleotide phosphate (NADP+) (46). cADPR, ADPR and NAADP bind different receptors and channels involved in the regulation of cytoplasmic Ca2+ fluxes, activating signaling pathways critical for different biological processes (for example, from lymphocyte activation [47,48] to glucose-induced insulin release in pancreas [49]).
CD38 is the major NAD glycohydrolase (NADase) in mammalian cells, regulating extracellular NAD+ levels. The family of NAD+-consuming enzymes includes four classes of molecules, of which CD38 and ADP ribosyl transferases (ARTs) are outside the cell, while PARPs and sirtuins are inside the cell. While consuming NAD+ as a substrate, these enzymes generate nicotinamide, reinforcing the notion that CD38 is directly involved in maintaining NAD+ homeostasis (50). Furthermore, extracellular NAD+ has been attributed novel additional roles in the control of immunity and of the inflammatory response and in shaping the regulatory T-cell compartment (51,52).
Generation of adenosine (ADO) occurs via the CD39 (ectonucleoside triphosphate diphosphohydrolase 1 [ENTPD1])/CD73 (ecto-5′-nucleotidase [5′-NT]) pathway. We hypothesized that CD38 may operate in conjunction with PC-1 (CD203a, pyrophosphatase/phosphodiesterase 1, ENPP1) to generate adenosine monophosphate (AMP), which is metabolized to ADO by CD73. The main characteristic of this pathway is that it works independently of the presence of CD39. The hypothesis was validated in normal cells (NK cells, Treg lymphocytes, mesenchymal stem cells and placenta) and in tumoral cells (myeloma and melanoma, among others) (Fabio Morandi, AL Horenstein, A Chillemi, G Zaccarello, V Quarona, Sabrina Chiesa, Marco Gattorno, F Malavasi and Vito Pistoia, unpublished data). The results obtained so far confirm that CD38/PC-1/CD73 is an important pathway of ADO generation and might be the production path in selected cells and tissues (AL Horenstein, A Chillemi, Fabio Morandi, G Zaccarello, V Quarona, Andrea Zito and F Malavasi, unpublished data). The pathway is sketched out in Figure 7.
Schematic representation of the CD38/PC-1/CD73 axis, which runs the production of ADO along with different products. NAM, nicotinamide; NAMPT, nicotinamide phosphoribosyltransferase; NMN, nicotinamide mononucleotide; NMNAT, NMN-adenylyltransferase; PRPPi, phosphoribosylpyrophosphate; TNAP, tissue nonspecific alkaline phosphatase.
The identification of the CD38/PC-1/CD73 pathway resulted from reconsidering old and new observations acquired independently on these individual ectoenzymes. Namely, PC-1 (clustered as CD203a) is a cell surface molecule that was identified originally as a murine plasma cell marker, for which it was accordingly named. PC-1 is an ectoenzyme and exerts ENPP1 functions. Also, PC-1 has multiple substrates with different catalytic efficiencies. Relevant to our context, ADPR, the product of the reactions controlled by CD38, is a substrate for PC-1 (53,54). When ADPR engages PC-1, the main products of the reaction are AMP, inorganic pyrophosphate (PPi) and ribose-1 phosphate. PPi is an inhibitor of calcification and bone formation. In the presence of alkaline phosphatase, it is transformed into inorganic phosphate (Pi). In addition, another substrate for PC-1 is adenosine 5′-triphosphate (ATP). After using either ADPR or ATP, PC-1 generates AMP to be used by CD73. The final product is ADO, along with Pi.
The CD38/PC-1/CD73 pathway may tip the balance from activation to anergy and suppression. A reaction initiated on the myeloma surface may produce AMP used by surrounding T and NK cells, inducing the production of ADO. These effects are likely more pronounced in closed systems (within lymph nodes, in spaces surrounding tumors, among the others) and less efficient in the blood stream, as shown in CLL (55).
An important consequence of this observation concerns the interactions with bone. Indeed, CD38 is expressed by myeloma cells, while PC-1 seems to be expressed by osteoblasts and stromal cells, where interaction with bone cells is critical for tumor growth (56). The same may be true in leukemic cells or solid tumors during metastasization. Engagement of CD38 by appropriate blocking mAbs may induce an interruption of the process leading to ADO, with effects on the tumor side or on the surrounding Tregs.
Conclusion
It is generally agreed that the CD38 glycoprotein is a particularly attractive target on malignant plasma cells at all stages of disease and in CLL patients with a poor clinical prognosis. In contrast, the molecule is detectable only at very low levels in mature lymphocytes or in nonhematopoietic tissues. This explains why three different companies (GenMab, now Johnson & Johnson, Sanofi and Morphosys) have developed human anti-human CD38 mAbs, all of which are now in early-stage trials in myeloma therapy (57). The same target also may find clinical applications in CD38+ CLL patients, whose prognosis is generally poor. Murine anti-CD38 mAb as F(ab′)2 or Fab could be used for arming drugs or nanoparticles with payloads varying according to the therapeutic strategies adopted, although this consideration lies outside the scope of this study.
Safety issues need to be considered for in vivo applications of anti-CD38 mAbs, whether used alone or for directing drugs or carriers. The information derived from tissue distribution and from pioneer experience in vitro indicates that early precursors of the hematopoietic stem cells do not express CD38 (58). However, tissue distribution may still be an issue, since the molecule is expressed in organs that have not yet been considered. One of these is the eye, where CD38 (along with its paralogue CD157) is expressed in areas involved in organ homeostasis (59).
The potential of anti-CD38 mAb therapy does not rest on the cytotoxic capacity of the specific immunoglobulin alone. The fact that CD38 is normally expressed outside the tumor cells may be irrelevant in and of itself, as experience with anti-CD20 mAbs shows. On the tumor side, surface density of CD38 target is easily upmodulable with retinoids. The effects are appreciable on myeloma and markedly on CD38+ CLL cells, and may constitute a significant resource for mAb-mediated therapy (60), not yet tested.
Another note of caution is that mAb ligation may induce activation signals on target cells. Gene profiling analysis has shown that these effects are greatly reduced in myeloma cells. Similar analysis performed by testing miRNAs has shown a reproducible increase of miR-33a-5p and miR-340-5p, whereas miR-193-5p and miR-629-3p are decreased. The induced miRNAs share the ability to influence signals involved in proliferation, cell cycle progression and migration (33,35,61). In any case, the safety issue is maintained and the limited number of miRNAs mobilized also may represent a beneficial effect.
The analysis of CD38 polymorphisms also may be useful in anticipating the efficiency of mAb treatment. A SNP located in intron 1 (rs6449182; C>G variation) differentially influences CD38 expression and its ability to be shed in biological fluids. This sequence is a binding site for the E2A transcription factor, providing one possible explanation for the observed effects (62). The obvious question is whether this and other polymorphisms may influence the synthesis, expression and release of CD38. Also worth investigating is whether different genotypes have the same ability to release CD38 in soluble forms or as exosomes, minicells where the molecule is maintained intact and functional. From this it follows that some individuals and patients would be more prone to release CD38 in biological fluids, a process that may divert mAbs from the real targets.
Lastly, a more general consideration is related to CD38 expression by tumor cells as a means of evading the immune response. The ectoenzyme CD38 may work in conjunction with PC-1/CD203a to generate AMP, which is metabolized by CD73 to ADO, a potent immunosuppressive agent. The doublet CD38/PC-1 expressed by the tumor may generate substrates metabolized by CD73 on the surface of the tumor itself or of the surrounding defense leukocytes. In this context, the recent findings of CD38 as a marker demarcating regulatory from memorylike T cells acquire a peculiar functional significance (63). An important point is that this pathway of ADO generation is independent from the presence of CD39. When occurring in closed systems (for example, tumor microenvironment or lymph nodes), local anergy and enhancement of tumor diffusion may ensue (64,65).
Moreover, CD38 is overexpressed by the majority of acute lymphoblastic leukemias, as well as by some cases of acute myeloid leukemias and non-Hodgkin lymphomas. It is reasonable to expect anti-CD38 mAbs to become a valuable tool in the treatment of a broad range of hematological tumors. Added values are that mAb-mediated therapy may be used directly, as a carrier of drugs or to redirect nanovescicles, and could be applied to maintenance, or in association with other drugs.
Disclosure
The authors declare they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Maecker HT, McCoy JP, Nussenblatt R. (2012) Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 12:191–200.
Alessio M, et al. (1990) CD38 molecule: structural and biochemical analysis on human T lymphocytes, thymocytes, and plasma cells. J. Immunol. 145:878–84.
Mallone R, et al. (1998) Characterization of a CD38-like 78-kilodalton soluble protein released from B cell lines derived from patients with X-linked agammaglobulinemia. J. Clin. Invest. 101:2821–30.
Hara-Yokoyama M, et al. (2012) Tetrameric interaction of the ectoenzyme CD38 on the cell surface enables its catalytic and raft-association activities. Structure. 20:1585–95.
Zhao YJ, Lam CM, Lee HC. (2012) The membrane-bound enzyme CD38 exists in two opposing orientations. Sci. Signal. 5:ra67.
Funaro A, et al. (1990) Involvement of the multi-lineage CD38 molecule in a unique pathway of cell activation and proliferation. J. Immunol. 145:2390–6.
Terhorst C, et al. (1981) Biochemical studies of the human thymocyte cell-surface antigens T6, T9 and T10. Cell. 23:771–80.
Fedele G, et al. (2004) CD38 is expressed on human mature monocyte-derived dendritic cells and is functionally involved in CD83 expression and IL-12 induction. Eur. J. Immunol. 34:1342–50.
Buggins AG, et al. (2010) Interaction with vascular endothelium enhances survival in primary chronic lymphocytic leukemia cells via NF-kappaB activation and de novo gene transcription. Cancer Res. 70:7523–33.
Ausiello CM, Urbani F, la Sala A, Funaro A, Malavasi F. (1995) CD38 ligation induces discrete cytokine mRNA expression in human cultured lymphocytes. Eur. J. Immunol. 25:1477–80.
Deaglio S, et al. (1998) Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J. Immunol. 160:395–402.
Horenstein AL, Stockinger H, Imhof BA, Malavasi F. (1998) CD38 binding to human myeloid cells is mediated by mouse and human CD31. Biochem. J. 330 (Pt 3):1129–35.
Liu Q, et al. (2005) Crystal structure of human CD38 extracellular domain. Structure. 13:1331–9.
Malavasi F, et al. (2008) Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 88:841–86.
Funaro A, et al. (1996) Identification and characterization of an active soluble form of human CD38 in normal and pathological fluids. Int. Immunol. 8:1643–50.
Zumaquero E, et al. (2010) Exosomes from human lymphoblastoid B cells express enzymatically active CD38 that is associated with signaling complexes containing CD81, Hsc-70 and Lyn. Exp. Cell. Res. 316:2692–706.
Damle RN, et al. (1999) Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 94:1840–7.
Chiorazzi N. (2012) Implications of new prognostic markers in chronic lymphocytic leukemia. Hematology Am. Soc. Hematol. Educ. Program. 2012:76–87.
Malavasi F, et al. (2011) CD38 and chronic lymphocytic leukemia: a decade later. Blood. 118:3470–8.
Funaro A, et al. (1998) CD38 functions are regulated through an internalization step. J. Immunol. 160:2238–47.
Dianzani U, Malavasi F. (1995) Lymphocyte adhesion to endothelium. Crit. Rev. Immunol. 15:167–200.
Newman PJ. (1999) Switched at birth: a new family for PECAM-1. J. Clin. Invest. 103:5–9.
Deaglio S, et al. (1996) Human CD38 ligand. A 120-KDA protein predominantly expressed on endothelial cells. J. Immunol. 156:727–34.
Deaglio S, et al. (2000) CD38/CD31, a receptor/ligand system ruling adhesion and signaling in human leukocytes. Chem. Immunol. 75:99–120.
Deaglio S, et al. (2007) CD38/CD19: a lipid raft-dependent signaling complex in human B cells. Blood. 109:5390–8.
Hu H, Li S, Liu J, Ni B. (2012) MicroRNA-193b modulates proliferation, migration, and invasion of non-small cell lung cancer cells. Acta Biochim. Biophys. Sin. (Shanghai). 44:424–30.
Chen J, et al. (2010) MicroRNA-193b represses cell proliferation and regulates cyclin D1 in melanoma. Am. J. Pathol. 176:2520–9.
Rauhala HE, et al. (2010) miR-193b is an epigenetically regulated putative tumor suppressor in prostate cancer. Int. J. Cancer. 127:1363–72.
Li XF, Yan PJ, Shao ZM. (2009) Downregulation of miR-193b contributes to enhance urokinase-type plasminogen activator (uPA) expression and tumor progression and invasion in human breast cancer. Oncogene. 28:3937–48.
Xu C, et al. (2010) MicroRNA-193b regulates proliferation, migration and invasion in human hepatocellular carcinoma cells. Eur. J. Cancer. 46:2828–36.
Gao XN, et al. (2011) MicroRNA-193b regulates c-Kit proto-oncogene and represses cell proliferation in acute myeloid leukemia. Leuk. Res. 35:1226–32.
Unno K, Zhou Y, Zimmerman T, Platanias LC, Wickrema A. (2009) Identification of a novel microRNA cluster miR-193b-365 in multiple myeloma. Leuk. Lymphoma. 50:1865–71.
Cirera-Salinas D, et al. (2012) Mir-33 regulates cell proliferation and cell cycle progression. Cell Cycle. 11:922–33.
Najafi-Shoushtari SH, et al. (2010) MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 328:1566–9.
Wu ZS, et al. (2011) miR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met. Cancer. 117:2842–52.
Deaglio S, et al. (2010) CD38/CD31 interactions activate genetic pathways leading to proliferation and migration in chronic lymphocytic leukemia cells. Mol. Med. 16:87–91.
Ferrero E, Saccucci F, Malavasi F. (1999) The human CD38 gene: polymorphism, CpG island, and linkage to the CD157 (BST-1) gene. Immunogenetics. 49:597–604.
Drach J, et al. (1994) Retinoic acid-induced expression of CD38 antigen in myeloid cells is mediated through retinoic acid receptor-alpha. Cancer Res. 54:1746–52.
Aggarwal S, et al. (2006) Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol. Biol. Cell. 17:566–75.
Uruno A, et al. (2011) All-trans retinoic acid and a novel synthetic retinoid tamibarotene (Am80) differentially regulate CD38 expression in human leukemia HL-60 cells: possible involvement of protein kinase C-delta. J. Leukoc. Biol. 90:235–47.
Le May N, et al. (2012) Poly (adp-ribose) glycohydrolase regulates retinoic acid receptor-mediated gene expression. Mol. Cell. 48:785–98.
Howard M, et al. (1993) Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 262:1056–9.
Kim H, Jacobson EL, Jacobson MK. (1993) Synthesis and degradation of cyclic ADP-ribose by NAD glycohydrolases. Science. 261:1330–3.
Zocchi E, Franco L, Guida L, Calder L, De Flora A. (1995) Self-aggregation of purified and membrane-bound erythrocyte CD38 induces extensive decrease of its ADP-ribosyl cyclase activity. FEBS Lett. 359:35–40.
Takasawa S, et al. (1993) Synthesis and hydrolysis of cyclic ADP-ribose by human leukocyte antigen CD38 and inhibition of the hydrolysis by ATP. J. Biol. Chem. 268:26052–4.
Aarhus R, Graeff RM, Dickey DM, Walseth TF, Lee HC. (1995) ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J. Biol. Chem. 270:30327–33.
Guse AH, et al. (1999) Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature. 398:70–3.
Morra M, Zubiaur M, Terhorst C, Sancho J, Malavasi F. (1998) CD38 is functionally dependent on the TCR/CD3 complex in human T cells. FASEB J. 12:581–92.
Takasawa S, Nata K, Yonekura H, Okamoto H. (1993) Cyclic ADP-ribose in insulin secretion from pancreatic beta cells. Science. 259:370–3.
Malavasi F, et al. (2010) The hidden life of NAD+-consuming ectoenzymes in the endocrine system. J. Mol. Endocrinol. 45:183–91.
Hubert S, et al. (2010) Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J. Exp. Med. 207:2561–8.
Chiarugi A, Dolle C, Felici R, Ziegler M. (2012) The NAD metabolome—a key determinant of cancer cell biology. Nat. Rev. Cancer. 12:741–52.
Katada T, et al. (2000) Enzymic and signal transduction properties of CD38/NADase and PC-1/phosphodiesterase. Chem. Immunol. 75:60–78.
Goding JW, et al. (1998) Ecto-phosphodiesterase/pyrophosphatase of lymphocytes and non-lymphoid cells: structure and function of the PC-1 family. Immunol. Rev. 161:11–26.
Serra S, et al. (2011) CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood. 118:6141–52.
Giuliani N, et al. (2012) Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia. 26:1391–401.
Flemming A. (2012) Deal watch: J&J and Genmab deal to push forward CD38 as a blood cancer target. Nat. Rev. Drug Discov. 11:822.
Verfaillie CM, Miller JS. (1994) CD34+/CD33− cells reselected from macrophage inflammatory protein 1 alpha+interleukin-3—supplemented “stroma-noncontact” cultures are highly enriched for long-term bone marrow culture initiating cells. Blood. 84:1442–9.
Horenstein AL, et al. (2009) CD38 and CD157 ectoenzymes mark cell subsets in the human corneal limbus. Mol. Med. 15:76–84.
Lane D. (2006) Designer combination therapy for cancer. Nat. Biotechnol. 24:163–4.
Gennarino VA, et al. (2012) Identification of microRNA-regulated gene networks by expression analysis of target genes. Genome Res. 22:1163–72.
Saborit-Villarroya I, et al. (2011) E2A is a transcriptional regulator of CD38 expression in chronic lymphocytic leukemia. Leukemia. 25:479–88.
Bahri R, Bollinger A, Bollinger T, Orinska Z, Bulfone-Paus S. (2012) Ectonucleotidase CD38 demarcates regulatory, memory-like CD8+ T cells with IFN-gamma-mediated suppressor activities. PLoS One. 7:e45234.
Zhang B. (2012) CD73 promotes tumor growth and metastasis. Oncoimmunology 1:67–70.
Stagg J. (2012) The double-edge sword effect of anti-CD73 cancer therapy. Oncoimmunology 1:217–8.
Pegoraro L, et al. (1989) The human myeloma cell line LP-1: a versatile model in which to study early plasma-cell differentiation and c-myc activation. Blood. 73:1020–7.
Quarona V, et al. (2013) CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin. Cytom. 2013, Apr 10. [Epub ahead of print].
Scatolini M, et al. (2010) Altered molecular pathways in melanocytic lesions. Int. J. Cancer. 126:1869–1881.
Ferracin M, et al. (2011) MicroRNA profiling for the identification of cancers with unknown primary tissue-of-origin. J. Pathol. 225:43–53.
Acknowledgments
This work was supported by grants from PRIN (Ministry of Education, University, and Research), from FIRB (Fondo per gli Investimenti della Ricerca di Base), from “ex-60%” Program (University of Torino) and from AIRC (Ig 13119 and partly from AIRC 5×1000). Antonella Chillemi and Valeria Quarona are students of the Ph.D. Program in Biomedical Sciences and Oncology at the University of Torino, Torino, Italy. Ada Funaro and Erika Ortolan provided expert assistance in the experiments of internalization and confocal analysis. The contributions of Silvia Deaglio, who provided samples of CLL patients, and Salvatore Oliviero, who contributed a preliminary analysis of the methylation of selected areas of the CD38 promoter, are gratefully acknowledged. Andrea Zito provided useful technical assistance, while Enrico Brunetti extensively reviewed the manuscript. The Fondazione Ricerca Medicina Sperimentale (FIRMS) assisted and supported this research project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit (https://doi.org/creativecommons.org/licenses/by-nc-nd/4.0/)
About this article
Cite this article
Chillemi, A., Zaccarello, G., Quarona, V. et al. Anti-CD38 Antibody Therapy: Windows of Opportunity Yielded by the Functional Characteristics of the Target Molecule. Mol Med 19, 99–108 (2013). https://doi.org/10.2119/molmed.2013.00009
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2013.00009