Abstract
The immunomodulatory effect of the autonomic nervous system has raised considerable interest over the last decades. Studying the influence on the immune system and the role in inflammation of the sympathetic as well as the parasympathetic nervous system not only will increase our understanding of the mechanism of disease, but also could lead to the identification of potential new therapeutic targets for chronic immune-mediated inflammatory diseases, such as rheumatoid arthritis (RA). An imbalanced autonomic nervous system, with a reduced parasympathetic and increased sympathetic tone, has been a consistent finding in RA patients. Studies in animal models of arthritis have shown that influencing the sympathetic (via α- and β-adrenergic receptors) and the parasympathetic (via the nicotinic acetylcholine receptor α7nAChR or by electrically stimulating the vagus nerve) nervous system can have a beneficial effect on inflammation markers and arthritis. The immunosuppressive effect of the parasympathetic nervous system appears less ambiguous than the immunomodulatory effect of the sympathetic nervous system, where activation can lead to increased or decreased inflammation depending on timing, doses and kind of adrenergic agent used. In this review we will discuss the current knowledge of the role of both the sympathetic (SNS) and parasympathetic nervous system (PNS) in inflammation with a special focus on the role in RA. In addition, potential antirheumatic strategies that could be developed by targeting these autonomic pathways are discussed.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a common, immune-mediated inflammatory disease affecting about 1% of the adult population worldwide. RA is characterized by inflammation of the synovium leading to progressive destruction of cartilage and bone (1,2). Although its exact etiology currently remains unknown, advances in understanding the pathogenesis and underlying mechanisms have led to the development of new and more effective antirheumatic drugs. Despite these improvements, a significant number of RA patients do not respond to the current therapies, and a need for identification of new pathways involved in modulation of inflammation to develop new antiinflammatory treatments still remains.
One such approach could be via manipulation of the autonomic nervous system. The nervous system is divided into the peripheral nervous system, with the sensory, somatic (voluntary) and autonomic (involuntary) sections, and the central nervous system. Classically, the autonomic nervous system is further divided into the sympathetic (SNS) and parasympathetic nervous system (PNS), which are in tight equilibrium. These systems typically act in opposition to each other, but are able to function in synergy, making it difficult to predict the effects of autonomic nervous system activation. In general, stimulation of the SNS brings the body to a state of raised activity and attention, usually called the fight or flight response: heart rate and blood pressure increase, liver glycogen is converted into glucose and peristalsis of the gastrointestinal tract is temporarily inhibited. In contrast, stimulation of the PNS can be summarized as the rest and digest response, as this returns the body functions back to normal: blood pressure lowers, heart rate slows down, gastrointestinal peristalsis is turned on again and the liver starts producing new glycogen (3).
It has become apparent that the nervous system has multiple anatomical and physiological connections with the immune system. Through these pathways, the nervous and immune systems have extensive communications using neurotransmitters, cytokines and endocrine hormones (4). Consequently, the nervous system is able to detect and regulate inflammation in peripheral tissues and is involved in maintaining immune homeostasis. An overview of the role of the autonomic nervous system in inflammation is shown in Figure 1.
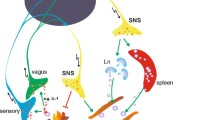
The role of the autonomic nerve system in rheumatoid arthritis. The autonomic nervous system is divided into the sympathetic (left) and parasympathetic (right) nervous system. Cell bodies of preganglionic sympathetic neurons are located in the central nervous system, between the first thoracic (Th1) and third lumbar (L3) spinal cord segments, from where its axon connects to the postganglionic neurons. Parasympathetic preganglionic neurons are found in the brainstem and the second through fourth sacral (S2–S4) spinal cord segments. The parasympathetic nervous system mainly consists of the vagus nerve, the tenth cranial nerve. The heart (A) is innervated by both the sympathetic and parasympathetic nervous system. Heart rate variability (HRV) is a technique to determine the balance of the autonomic nervous system. In rheumatoid arthritis patients, this balance can be altered compared with healthy individuals. This imbalance is caused by a dominant influence of the sympathetic nervous system instead of the parasympathetic nervous system, which under normal conditions, to a great extent, determines HRV. Both lymphoid organs (spleen and lymph nodes) (C) and joints (D) are innervated directly by the sympathetic nervous system, but no innervation of the vagus nerve (parasympathetic nervous system) has been found. Stimulating the vagus nerve and thereby reducing inflammation (a concept known as the cholinergic antiinflammatory pathway) does, however, need the presence of the spleen. Fibers of the vagus nerve end in the celiac-superior mesenteric plexus (B), further innervation of the spleen via the splenic nerve has been found to contain only catecholaminergic fibers from the sympathetic nervous system. It is not known how the vagus nerve can signal via the spleen without direct innervation, but it is proposed that the catecholaminergic splenic nerve transmits the parasympathetic signal toward the spleen. Inflammation is registered in the brain via the circulation, but also via the afferent vagus nerve (E). Subdiaphragmatic vagotomy inhibits signaling of inflammation toward the brain. As the joint is not innervated by the vagus nerve, most likely direct detection of joint inflammation is not possible.
Several observations have supported the notion that there may be an interaction between the nervous system and inflammation in RA. For instance, it has been reported that hemiplegic patients who develop RA do not develop arthritis on their hemiplegic (denervated) side (5). This eventually led to the hypothesis that experimental modulation of the body’s neural pathways involved in regulating inflammation could potentially lead to the development of new treatment options for different inflammatory diseases, including RA.
Sympathetic Nervous System
Functional Anatomy of the Sympathetic Nervous System
The autonomic nervous system runs from the central nervous system to the peripheral organs through two different neurons: the preganglionic and the postganglionic neurons. The cell body of a preganglionic neuron is located in the central nervous system, between the first thoracic (Th1) and third lumbar (L3) spinal cord segments, from where its axon connects to the postganglionic neuron. Subsequently, the axon of the postganglionic neuron projects to the target organ (6).
All regions of the body receive sympathetic innervation. This enables the SNS, together with the hypothalamic-pituitary-adrenal (HPA) axis, to be a key peripheral regulator in maintaining internal homeostasis. Although discussion of the HPA axis is beyond the scope of this review, it must be mentioned that this axis and the SNS are involved in a mutual, positive feedback loop and activation of one system usually activates the other as well (7). When homeostasis is disturbed, both the HPA axis and the SNS become activated to restore the internal milieu.
Catecholamines, such as epinephrine (adrenaline), norepinephrine (noradrenaline, NE) and dopamine, are released by the adrenal gland or nerve tissue and act as sympathomimetic hormones or neurotransmitters. In response to a stimulus, most sympathetic postganglionic neurons release NE, which subsequently activates adrenergic receptors (ARs) on the peripheral target tissue. There are two adrenergic receptor types (α- and β-AR), and both exhibit a different affinity for NE, depending on the receptor subtype (α1, α2, α3, β1 or β2) and the tissue where they are expressed (8). The effect of NE on the different receptors also depends on the receptor’s local concentration in the synaptic cleft: a high NE concentration (1–100 µmol/L) acts on both α- and β-ARs, but at low concentrations (≤0.1 µmol/L), NE predominantly stimulates α-ARs (9).
General Role of Sympathetic Nervous System in Inflammation
The regulatory function of the SNS includes monitoring and influencing immune homeostasis. During an immune response, peripherally secreted proinflammatory cytokines, such as interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-α, can signal to the brain in two ways: via the circulation (4) or through afferent fibers of the vagus nerve (10,11) (also see Role of Vagus Nerve: Cholinergic Antiinflammatory Pathway below). This can result in central activation of the SNS, which, in turn, stimulates catecholamine production in the different lymphoid organs via the efferent, sympathetic fibers (4,7).
Both primary (bone marrow, thymus) and secondary (spleen, lymph nodes, mucosa-associated lymphoid tissue) lymphoid organs are innervated extensively by the SNS (7,8,12). Interestingly, the distribution and density of sympathetic nerves in lymphoid organs are not stable, but appear to change in response to alterations in the microenvironment, including immune activation and changes in cytokine release (12–14). The immune system is thereby capable of influencing the SNS and the innervation of lymphoid organs allows the SNS to influence the immune cells directly, via released NE which binds to ARs on these cells.
Cells of the adaptive immune system primarily express the β2-subtype, whereas cells of the innate immune system appear to express not only β2-, but also α1- and α2-ARs (8,12). Stimulation of these subtype receptors on macrophages can elicit different functional effects. Macrophages can be activated through α2-AR stimulation, while stimulation of the β2-AR has a suppressive effect on macrophage activity (12). For example, during late-stage sepsis, β2-ARs were upregulated on rat liver Kupffer cells, a macrophage subset, leading to inhibition of Kupffer cell function and subsequent immunosuppression (15). Thus, not only the density of SNS fibers, but also the AR binding capacity on immune cells, is adaptive to environmental needs. In addition, immune function is regulated by peptide neurotransmitters that colocalize with NE, such as neuropeptide Y and adenosine triphosphate (ATP), as well, but their exact contribution to immune regulation remains to be unraveled (12).
The role of SNS activation in immune cell functioning is complex. In vitro and in vivo studies on the effects of NE, or different adrenergic agonists and antagonists on immune cells report dissimilar results (8,12). In short, NE’s effects during an immune reaction appear to be dependent on which immune cells are present, the activation state of these cells, the type and number of ARs they express (see above), and the surrounding cytokine context. Most evidence points toward the fact that SNS activity is able to inhibit the development of a T helper (Th)1 immune response and shift the Th1/Th2 balance toward a Th2 immune response (7,12). Besides influence on proliferation of immune cells and their cytokine production, the SNS also appears to be involved in regulation of leukocyte trafficking to sites of inflammation (16). The exact mechanism by which the SNS or adrenergic agents influence cell migration is as yet unknown, but this might be due to the sympathetic influence on vascular smooth muscle regulating the blood flow and thereby the lymphocyte delivery (8). It also has been shown that stress leads to changes in expression of cell adhesion molecules on immune cells (17,18), thereby possibly influencing cell migration from the blood to the site of inflammation.
Sympathetic Nervous System and Arthritis
In general, RA patients have an autonomic imbalance with an overly active SNS and reduced PNS activity (19). Determining the heart rate variability with an electrocardiogram is an established technique to evaluate this autonomic balance. A low variability in heart rate reflects an increase in SNS activity and reduced PNS activity (Figure 1A). In established RA, it has been suggested that stress may aggravate disease activity. Minor stress (lasting from minutes to a few days with small intensity), that is, daily hassles, may lead to a short rise of neurotransmitters and hormones, and could increase disease activity in RA patients (20). The role of the stress response is however far from clear. The Lewis rat, for instance, has a defective stress response system and is much more sensitive to the development of arthritis than F344 rats, which are characterized by a hyperactive SNS (21).
β2-Adrenergic Receptor Expression and Function in Rheumatoid Arthritis
β2-ARs have been shown to have a role in the time-dependent, immunomodulating effect of the SNS (22–24) and they are expressed on innate and adaptive immune cells of humans and rodents (7). Peripheral blood mononuclear cells (PBMCs), specifically monocytes, B cells and CD8+ T cells, from RA patients express lower levels of β2-ARs compared with healthy subjects (25) and the receptor density on RA synovial fluid lymphocytes is even less (26). RA PBMCs therefore also are less responsive to NE treatment compared with healthy subjects: upon NE administration in vitro (25,27) they are less effective in suppressing T-cell activation and proliferation, and the stimulated cytokine response was not decreased as much. In active systemic and polyarticular juvenile RA there is a diminished cAMP production upon β2-AR stimulation, indicating a defective neuroimmune response (28).
a-Adrenergic Receptor Expression and Function in Rheumatoid Arthritis
The role of α-AR subtypes in arthritis is less clear. As mentioned above, only cells from the innate immune system appear to express α-ARs (8,12). Normally, α1-ARs are expressed only on natural killer cells, whereas α2-ARs are present on natural killer cells, monocytes and macrophages (12). However, in vitro studies showed that treating normal monocytes with β2-AR agonists can induce expression of the α1-AR subtype (29). On the contrary, in RA patients with high disease activity, catecholamines mainly mediate their effect through α1-ARs on PBMCs (30,31), while showing a decreased density of β2-ARs (25,31). Functional α1-ARs also appeared to be upregulated on leukocytes of juvenile idiopathic arthritis patients, while being absent on leukocytes from healthy donors, and stimulation of these receptors induced higher IL-6 levels (32). Furthermore, stimulation of α2-ARs can initiate proliferation of fibroblast-like synoviocytes (FLS) in the joint (12,30), which will lead to increased production of cytokines, proteases and chemokines. Subsequently, more immune cells may be recruited to the joint, eventually resulting in the cartilage destruction typically seen in RA. It has been suggested that α-ARs might become more relevant in a later stage of chronic inflammation, coinciding with decreased numbers of β-ARs. This observation has been called the β-to-α-adrenergic shift (30).
Immunomodulatory Effect of Agents Targeting the Sympathetic Nervous System in Animal Models of Arthritis
Intervening with SNS activity may influence the inflammatory process seen in RA, either locally or systemically. To date, clinical testing of AR agents as a treatment option for RA patients has not been performed and our current knowledge is based on data obtained in animal models of arthritis. In the sections below, the results of these studies are discussed and in Table 1 the available experiments are summarized.
Time-dependent effect of adrenergic intervention. Studies with AR drugs in animal models of arthritis showed conflicting results: both exacerbating and ameliorating effects of AR agonists on arthritis severity were seen (22,23,33,34). This might be due to the timing of drug administration, and it is now well established that the SNS induces time-dependent, immunomodulating effects in arthritis models: prior to arthritis induction and in the asymptomatic phase, SNS activation has a proinflammatory effect, whereas, after onset of arthritis, SNS activation may inhibit inflammation (24,30,35). This was nicely shown in a collagen-induced arthritis (CIA) mouse model in mice in which chemical sympathectomy (leading to a 75% reduction in NE production) was initiated by intraperitoneal (i.p.) injections with 6-hydroxydopamine (6-OHDA) at different time points (35) (see also Table 1). The transition phase from pro- to anti-inflammatory effects by the SNS is thought to lie between day 30 and day 55 after immunization in murine CIA (14,35). It has been suggested that the early, proinflammatory effects are caused by SNS-induced redistribution, migration and chemotaxis of leukocytes toward the site of inflammation (30,35). Interestingly, the influence of the SNS on CD4+CD25+ T cells seems to have a role in arthritis severity. SNS intact, splenectomized (to minimize endogenous CD4+ T cells), recipient CIA mice received either CD4+CD25+ T cells from SNS-intact CIA mice or from sympathectomized CIA mice. The CD4+CD25+ T cells that were influenced by an intact SNS promoted arthritis compared with the SNS-deprived CD4+CD25+ T cells. Thus, it appears likely that the SNS has an effect on the FoxP3- subset of proinflammatory CD4+CD25+ T cells. The CD4+CD25+ T cells from the sympathectomized CIA mice also displayed a reduced cytokine response (36).
β2-Adrenergic receptor agonists or antagonists in experimental arthritis. To our knowledge, no studies on the effect of β1-agonists on experimental arthritis have been performed. β2-ARs presumably play an important role in the time-dependent, immunomodulating effect of the SNS. In studies that used the adjuvant-induced arthritis (AIA) model in rats, treatment with β2-AR agonists resulted in worsening of disease severity, when this was initiated prior to or at the time of adjuvant challenge (22,24), while administration of β2-AR antagonists started prior to adjuvant challenge significantly reduced the severity of joint injury and delayed disease onset (22,23). Furthermore, initiating β2-AR agonist treatment at or after onset of disease also led to a reduction in disease severity (24) (Table 1).
α-Adrenergic receptor agonists or antagonists in experimental arthritis. In vivo studies on the function of α-AR subtypes in experimental arthritis have shown opposing results. Blocking with a nonspecific antagonist of α-AR (both α1-and/or α2-AR) has a different effect on disease severity depending on the time that the agent is given (23,24). If administrated at disease onset, it reduces the severity of disease, but aggravation of disease severity is observed when given prior to immunization (24). Specific blocking of the α1-AR with an antagonist prior to disease onset did not result in an effect on experimental arthritis (23,33), but using an α2-AR antagonist does increase arthritis severity in experimental arthritis in Sprague-Dawley rats (33). The aggravation of arthritis when treated with a nonspecific α-AR antagonist, when given before immunization, might, therefore, be the result of an effect via the α2-AR (23,24).
Treatment with specific α2-AR agonists, given before disease onset, has a positive effect on arthritis (33). Nothing is known about the effect of specific α1-AR agonists on arthritis activity in animal models.
Sympathetic Innervation of the Spleen and Joints in Experimental and Rheumatoid Arthritis
Nervous innervation of the rodent and human spleen currently is believed to be primarily by SNS fibers, as no cholinergic fibers have been identified here so far (8,37) (Figure 1C). In spleens of arthritic rats (AIA), the nerve density in white pulp distal to the hilus is reduced considerably, while nerve density in the hilus and red pulp is increased (38). The red pulp, the site where activated immune cells reside and eventually exit the spleen (12), is normally sparsely innervated by sympathetic fibers, but a sympathectomy resulted in a compensatory sprouting of noradrenergic nerves in red pulp of arthritic, but not of nonarthritic rats (38). The investigators suggested that this altered sympathetic innervation pattern in the red pulp was thought to reflect a regulated microenvironment, where migrated immune cells provide trophic support to the redistributed SNS fibers, which, in turn, could play a critical role in sustaining immune dysregulation seen in chronic inflammatory stages of arthritis (38). In addition, an overall reduction of sympathetic nerve fibers was found in the spleen of DBA/1 mice with early, symptomatic CIA compared with normal DBA/1 mice. Despite this reduction, the SNS was still able to stimulate interferon-γ and cytokine-induced neutrophil chemoattractant-1 (CXCL-1) secretion by splenic T cells via various neurotransmitter systems (14). This demonstrates that the SNS can still contribute to disease-aggravating effects in the early phase of arthritis despite reduced fiber density (14). As far as we are aware, there are no data on altered sympathetic innervation density of the spleen in RA patients.
Sympathetic innervation of the joint is decreased largely in experimental arthritis, as was shown during the symptomatic stages of disease in murine CIA (39). Similarly, the number of sympathetic nerve fibers in the joints of RA patients is reduced significantly, coinciding with an increased degree of inflammation, but also with significantly more substance P-positive nerve fibers and an augmented NE production by synovial cells (macrophages, B cells, fibroblasts, mast cells and granulocytes) (9,40,41). Moreover, in AIA rats, ankle joints that developed more severe arthritis were more densely innervated by substance P-containing, primary afferent neurons than were joints that developed less severe arthritis (knees) (42). Infusion of substance P into these knees did increase arthritis severity, suggesting that this neuropeptide also may contribute to inflammation in RA (9,42). This implies a diminished influence of the SNS on, and more involvement of sensory inputs in, established joint inflammation.
In longstanding RA, a marked loss of sympathetic nerve fibers can be found (9). After reduction of sympathetic innervation in the inflamed joint, the synovial tissue itself has a role in producing NE (9,40). The concentration of NE in synovial tissue is in the α-adrenergic region 10−9 to 10−8 mol/L, which is known mainly to have an activating effect on the α-ARs. Moreover, in patients with RA, who markedly lose sympathetic nerve fibers, the density of catecholamine-producing cells was higher than in patients with OA, who do not lose sympathetic innervation. Blockade of catecholamine storage into vesicles by reserpine caused a very strong and specific inhibition of TNF-α mRNA and TNF-α protein from cells of patients with OA and RA (41). In addition, the effect of cytoplasmic catecholamine increase was tested in vivo in CIA in mice. It was found that local treatment with reserpine markedly reduced inflammation without causing systemic side effects in the animals (41). This indicates that when sympathetic nerve fibers are lost in inflamed tissue, sympathetic cells can take over. These findings demonstrate that peripheral cells start producing catecholamines during chronic inflammation, and the increase of cytoplasmic catecholamines has strong antiinflammatory effects in vitro and in vivo. Therefore, modulation of catecholamine-producing cells could be used as a new therapeutic target in arthritis.
Parasympathetic Nervous System
Functional Anatomy of the Parasympathetic Nervous System
As mentioned in the introduction, activity of the PNS results in the rest and digest effect, enabling the body to restore its energy supplies. Parasympathetic, preganglionic neurons are found in the brainstem and in the second through fourth sacral (S2–S4) spinal cord segments (see Figure 1). The postganglionic neurons reside in parasympathetic ganglia on or near the target organs, in contrast to the sympathetic ganglia, which are located at greater distance from the target organs (6). The PNS consists mainly (75%) of the vagus nerve, the tenth cranial nerve (3), which is the largest nerve and owes its name to the wandering course it runs along the body (43). The efferent fibers of the vagus nerve originate mainly in the dorsal motor nucleus of the vagus and the nucleus ambiguous in the medulla oblongata (brainstem). From there, they pass through the neck and thorax to the abdomen, meanwhile branching off fibers to various organs, including the heart, lungs, gastrointestinal tract and pancreas (43,44).
Acetylcholine (ACh) is the main parasympathetic, postganglionic neurotransmitter, but it also is released in all preganglionic neurons, both parasympathetic and sympathetic. The enzyme choline acetyltransferase (ChAT) synthesizes ACh from choline and acetyl-CoA, and acetylcholinesterase (AChE) can degrade it into the inactive metabolites choline and acetate. ACh can bind to two types of receptors, the muscarinic (mAChR) and the nicotinic (nAChR) receptors, which are both widely expressed in neuronal as well as nonneuronal cells and can consist of different subunits. Many different nicotinic (α1–α10 and β1–β4) and muscarinic (M1–M5) subunits have been identified, leading to a wide variety of possible receptors with different physiological functions (45–47). In the context of rheumatoid and experimental arthritis not much is known about the role of various nicotinic (α1–α6, α8–α10) and muscarinic (M1–M5) receptors. Therefore, in this review we will focus specifically on the α7 nicotinic acetylcholine receptor (α7nAChR).
The nonneuronal cells that can produce ACh, for example, epithelial, endothelial, mesothelial, muscle, and immune cells, also possess all functional components of the cholinergic system (48) and the cholinergic signaling in these cells is comparable to regular neurotransmission (49). Furthermore, it has been suggested that dysfunction in these nonneuronal cholinergic systems might be involved in the pathogenesis of several chronic diseases, such as colitis ulcerosa, cystic fibrosis and psoriasis (49).
General Role of Parasympathetic Nervous System in Inflammation
How exactly the PNS and, in particular, the vagus nerve are involved in the immune system is still under investigation. A summarizing review from 2007 that covered 20 years of neuroanatomical research on the autonomic innervation of the immune system stated that there is no neuroanatomical evidence for parasympathetic or vagal nerve innervation of any immune organs (8,50) (see Figure 1C). This conclusion was based on numerous studies that reported negative results for retrograde tracing from the immune organs to vagal brainstem nuclei, combined with absence of ACh, vesicular ACh transporter-labeled fibers and/or Ach-metabolizing enzymes, such as ChAT and AChE, in the immune organs. In addition, studies using electrical field stimulation of spleen slices indicated that sympathetic, but not cholinergic, nerve fibers innervate the spleen. (14). On the other hand, there was local ACh expression, but this could be explained theoretically by release from splenic immune cells. In spite of the apparent absence of direct vagal nerve innervation, however, there is evidence that the spleen receives not only sympathetic signals, but also parasympathetic input (51). Recent work has shown that the vagus nerve may inhibit TNF-α production by splenic macrophages via a signal from the celiac-superior mesenteric plexus projecting in the splenic nerve, which is comprised principally of catecholaminergic fibers (Figure 1B) (see section Route of the Cholinergic Antiinflammatory Pathway below) (52).
Role of Vagus Nerve: Cholinergic Antiinflammatory Pathway
Whether directly innervating immune organs or not, it has been widely acknowledged that the PNS, through the vagus nerve, plays an important role in regulation of inflammation. Stimulation of peripheral, afferent vagus nerve fibers by endotoxin or cytokines can activate the HPA axis and SNS centrally, resulting in peripheral release of antiinflammatory glucocorticoids and NE (7,53–57). In addition, it has been shown that efferent vagus nerve fibers are involved in inflammatory modulation, by performing bilateral cervical vagotomy in a rat model of experimental sepsis (58). Subsequent electrical stimulation of the peripheral part of the vagus nerve significantly decreased serum TNF-α levels and prevented development of shock compared with vagotomized rats that did not receive electrical stimulation. This peripheral vagus nerve stimulation (VNS) in the vagotomized animals was not followed by enhanced glucocorticoid production, indicating that the suppressed TNF-α production could not be attributed to activation of the HPA axis, but was achieved merely through the efferent vagus nerve (58). This has been confirmed in subsequent studies, where VNS significantly attenuated TNF-α synthesis and improved clinical outcome in experimental models of hemorrhagic shock (59,60) and ischemia-reperfusion injury (59,60), and blocked leukocyte migration by inhibiting endothelial cell activation, a key regulator of leukocyte trafficking during inflammation, in the carrageenan air pouch rat model (61). This antiinflammatory influence of the efferent vagus nerve is now well known as the cholinergic antiinflammatory pathway (62).
The α7 Nicotinic Acetylcholine Receptor
In a first attempt to determine how the efferent vagus nerve establishes its antiinflammatory effect, in vitro experiments studying the cytokine release by primary human macrophages showed that ACh dose-dependently inhibited release of TNF-α, IL-1β and IL-6 after 4 and 20 h of LPS stimulation, primarily through nAChRs; production of the antiinflammatory cytokine IL-10 was unaffected (58). Nicotinic AChRs, which are integral membrane proteins belonging to the ligand-gated, ion channel superfamily, are further subdivided into muscle-type nicotinic receptors, found at the neuromuscular junction, and neuronal-type nicotinic receptors. In humans, 17 different subunits have been identified (α1–10, β1–4, γ, δ, ε) and five transmembrane subunits are assembled around a central pore to form a homo- or heteromeric nAChR (63). Subsequent research led to the identification of the neuronal-type α7 nACh receptor subtype (α7nAChR) as the essential regulator of the antiinflammatory effect of ACh (64). Additional in vitro studies using human monocytes, PBMCs and whole blood showed that nicotine and the α7nAChR selective partial agonist GTS-21 dose-dependently inhibited the release of TNF-α and IL-1β after LPS stimulation, with GTS-21 being more potent than nicotine (64–66).
The α7nAChR consists of five α7 subunits that assemble to form a ligand-binding, homopentameric ion channel (67). The receptor is found not only on neurons and macrophages, but also is expressed widely on other nonneuronal cells, including immune cells, such as monocytes, T and B lymphocytes, dendritic cells (68) and RA FLS (69–71). In addition to this classical variant of the α7 receptor, the human genome contains an alternative, partial duplication of the α7 subunit gene, called the dupα7 or “cholinergic receptor family with sequence similarity to α7.” The main difference between this and the classical α7 is that this dupα7 subunit lacks binding sites for nicotine or other α7 agonists/antagonists and, to date, the role of the dupα7 remains unclear. In fact, although it is transcribed in different cells, including human leukocytes (72) and FLS (69), it is not clear whether this transcript is ever translated into a functional protein and eventually can form a functional receptor (68,72). It is possible that the dupα7 has a regulatory role, intervening or contributing to the function of the classical α7nAChR and therefore might provide new pharmacological options (69).
Several in vivo studies confirmed the importance of the α7nAChR in the cholinergic antiinflammatory pathway. Receptor activation by specific agonists effectively attenuated immune responses and ameliorated disease severity in different experimental settings, including animal models for sepsis (73,74), pancreatitis (75), ischemia-reperfusion injury (76), postoperative ileus (77), acute lung injury (73), and, as discussed in more detail below, CIA (78,79). Moreover, in α7-subunit-deficient mice, VNS failed to reduce serum TNF-α levels during endotoxemia (64).
Route of the Cholinergic Antiinflammatory Pathway
Not only the α7nAChR is essential in the cholinergic antiinflammatory pathway, the spleen is thought to play an important role as well in the regulation of systemic inflammation by the efferent vagus nerve, since electrical VNS failed to diminish serum TNF-α levels in splenectomized mice treated with endotoxin (80). As mentioned earlier, there is no convincing evidence that the spleen is actually innervated by the vagus nerve (see Figure 1B). In this light, a further study on unraveling the road of the cholinergic antiinflammatory pathway revealed that the nerve fibers found in apposition to the TNF-α-secreting macrophages in the spleen are catecholaminergic and not cholinergic (52). These catecholaminergic nerves proved to be required for functional inhibition of TNF-α production by VNS. It is thought that the vagus nerve functionally communicates with the splenic nerve at the level of the celiac and superior mesenteric ganglia which may lead to secretion of catecholamines, such as NE, by the splenic nerve (52). NE has been shown to have a direct, inhibitory effect on TNF-α secretion by splenic macrophages by acting on ARs (81,82). In addition, there is a direct inhibitory effect of ACh on TNF-α secretion by macrophages through activation of the α7nAChR (64). As the spleen contains and releases ACh upon splenic nerve stimulation (83,84), ACh is most likely produced by immune cells present in the spleen, which (also) attenuates cytokine production by signaling through α7nAChR on TNF-α-producing cells (52).
Interestingly, it was shown recently that GTS-21 can reduce serum TNF-α levels in both normal and splenectomized animals using an animal model of hemorrhagic shock (85). It is, therefore, conceivable that the cholinergic antiinflammatory pathway consists of two separate arms: (i) the inhibitory effect of ACh produced by nonneuronal cells on cytokine production by immune cells at the site of inflammation through α7nAChR activation and (ii) the immunosuppressive effect of the efferent vagus nerve by communicating with the splenic nerve via the celiac and superior mesenteric ganglia, and the subsequent stimulation of both α7nAChRs and ARs in the spleen.
Parasympathetic Nervous System in Experimental and Rheumatoid Arthritis
We have described recently the role of the cholinergic antiinflammatory pathway in murine CIA (78). Systemic treatment with nicotine or the α7nAChR specific agonist AR-R17779 significantly ameliorated arthritis activity, whereas unilateral cervical vagotomy exacerbated the disease. The effect of AR-R17779 appeared more potent than that of nicotine, pointing to an important role of the α7nAChR in mediating the antiinflammatory effect. Moreover, AR-R17779 hardly crosses the blood-brain barrier, indicating that these effects are achieved by stimulation of peripheral α7nAChRs. We also tested the pharmacological and functional profile of two novel compounds, CTI-15311 and CTI-15072, with different effects on ion channel activity and investigated their role in modulating CIA (unpublished results, van Maanen et al.). Although both compounds bound to α7nAChR with high affinity, CTI-15311 acted like a classical agonist of ion channel activity, whereas CTI-15072 did not produce an electrical current, suggesting it can act as an ion channel antagonist. Despite not being able to induce significant levels of ion channel activity, CTI-15072 could still improve arthritis, albeit to a lesser extent than CTI-15311. Moreover, CTI-15072 was clearly distinct from typical competitive antagonists, since it was able to synergize with the positive allosteric α7nAChR-modulator PNU-120596 to induce detectable ion channel current, suggesting that it is a selective desensitizer of α7nAChR. These data provide direct evidence that the α7nAChR on immune cells does not require typical ion channel activation to exert its antiinflammatory effects. Moreover, CTI-15072 only showed 6% brain penetration, indicating that the antiinflammatory effect on experimental arthritis via the α7nAChR can be observed despite limited penetration of the central nervous system.
In subsequent experiments, it was demonstrated that clinical arthritis scores and synovial inflammation were increased markedly in α7nAChR-deficient mice compared with their wild-type littermates in both the acute and chronic phase of the disease (86). These data suggest that the α7nAChR, and thereby the cholinergic antiinflammatory pathway, is involved in the regulation of arthritis activity in experimental arthritis. More evidence pointing toward a role for the α7nAChR in arthritis was obtained from experiments using whole blood from RA patients, who exhibited suppressed vagus nerve activity (87). Upon stimulation, inflammatory cells from these whole blood cultures produced less TNF-α as compared with healthy controls, but the addition of cholinergic agonists to the stimulated whole blood cultures still suppressed cytokine production significantly, implying that targeting the α7nAChR could indeed be a new treatment option in these patients (87).
Presence of the α7 Nicotinic Acetylcholine Receptor in the Joint
To date, there is no explicit evidence of vagal innervation of the joints (Figure 1D). However, we and others have shown that the α7nAChR is expressed in synovial tissue and on RA FLS. In vitro stimulation of the receptor (with ACh or α7nAChR agonists) suppressed TNF-α- and IL-1β-induced production of IL-6 and IL-8 by RA FLS (69,70). Furthermore, the presence of a nonneuronal cholinergic system in human synovial tissue was described recently. Synovial biopsies from RA and OA patients showed expression of ChAT in both mononuclear-like cells as well as FLS, indicating that ACh can be produced locally by these cells (88). This local ACh production might be important in the regulation of joint inflammation, thereby contributing to the cholinergic antiinflammatory pathway. Whether the release of nonneuronal ACh is triggered by neuronally released ACh or if the nonneuronal cholinergic system in synovial tissue acts independently from cholinergic nerves remains to be investigated further (89).
The regulation of immune function is certainly not bound to be exerted by ACh alone, but a potentiating effect of other endogenous cholinergic agonists may be conducive. One such agonist is SLURP-1 (secreted mammalian Ly6/urokinase plasminogen-type activator receptor-related protein-1), which is produced by both lymphocytes and macrophages, and has been shown to potentiate ACh-mediated α7nAChR activation (90).
Dysregulation of Autonomic Nervous System in Rheumatoid Arthritis
As discussed, both the SNS and PNS, and also the HPA axis, influence immune homeostasis. Inflammatory mediators signal to the brain via the circulation or via afferent fibers of the vagus nerve, thereby activating the SNS and/or PNS (Figure 1E). Efferent SNS and vagal nerve fibers then induce local catecholamine and ACh production by neurons or nonneuronal cells. The final effects are difficult to predict, as there are many different signaling molecules and receptors involved depending on the disease phase.
The autonomic dysfunction in RA patients is characterized by an increased overall sympathetic tone and decreased activity of the vagus nerve. This indicates that the normal equilibrium, where the SNS and PNS act oppositely and have contrary effects, is in imbalance and this disturbance might contribute to the induction and/or persistence of the disease (19,91–95). When immune homeostasis is disturbed, both the SNS and HPA axis are activated to restore this. In RA patients, however, inadequately low levels of cortisol were seen in relationship to inflammation and controlled physiological stress (96). These findings led to the assumption that the HPA axis and SNS have been uncoupled in these patients, resulting in a strong proinflammatory situation (30,96). Other changes also take place, for example, density of sympathetic nerve fibers in arthritic patients are decreased (14), while SNS fibers in the spleen redistribute toward the red pulp where the immune cells reside, suggesting there is an altered sympathetic-to-immune system signaling in RA.
Simultaneously, low tone of the vagus nerve means low activity of the cholinergic antiinflammatory pathway, which also can lead to higher cytokine levels, thereby contributing to this proinflammatory status. Of note, surgical unilateral vagotomy for gastric disease in humans had no effect on the risk of developing RA, but vagotomy at a different anatomical level might have resulted in a different result (97). When considering the parasympathetic-to-immune system signaling, our data show that the cholinergic antiinflammatory pathway contributes to control of disease activity in experimental arthritis, since interruption of this pathway can lead to aggravation of arthritis. Our findings support and extend the pioneering work by KJ Tracey, suggesting that tonic activity of the vagus nerve is essential to maintain immune homeostasis (62). Impairment of this activity could lead to unrestrained cytokine responses and damage to the host.
It is unknown whether the dysregulation of the autonomic nervous system is initiated by an increase in SNS activity (for example, stress) followed by decrease in vagus nerve activity, or if it is the other way around. Regardless, based on experimental results stated above, it seems likely that the autonomic imbalance observed in experimental arthritis and RA patients is, at least partially, responsible for sustaining the inflammatory status. Further insight into this autonomic dysregulation in RA is essential to determine how and to what extent it contributes to the development, persistence and exacerbation of arthritis.
Potential Clinical Implications For Modulation of the Nerve System in Inflammatory Diseases
The immunoregulatory potential of the SNS has led to the proposition of new antiinflammatory therapeutic approaches for RA. Based on animal models discussed above, it can be envisaged that intervening with SNS activity can influence inflammation in RA. Until now, no clinical testing of AR agents as a treatment option for RA patients has been performed. Since data targeting AR receptors obtained from animal models are somewhat variable, it is difficult to draw definitive conclusions as to which treatment would be beneficial in arthritis patients.
The PNS can exert its antiinflammatory effects via the vagal cholinergic antiinflammatory pathway in which the neurotransmitter acetylcholine plays an important role. The discovery of the cholinergic antiinflammatory pathway has paved the way for new therapeutic strategies for inflammation-mediated diseases like RA. The two major treatment options are pharmacological intervention or electric stimulation of the vagus nerve (VNS). The cholinergic antiinflammatory pathway can be stimulated by pharmacologic activation of the nAChRs. The observation that specific nAChRα7 agonists diminish disease in several animal models of inflammation, including animal models for arthritis (78,79) and experimental colitis (98), suggests that therapeutic agents that can modify cholinergic signaling might be beneficial in humans. Nicotine, a potent nAChRα7 agonist, has been used extensively to examine the cholinergic antiinflammatory pathway in animal models. However, the therapeutic value of nicotine is limited, because of its lack of specificity and toxicity. To avoid side effects caused by nicotine, more specific nAChR agonists have been designed. The most extensively characterized nAChR specific agonist is GTS-21, a nAChRα7 agonist that also affects 42 nAChR. GTS-21 is well tolerated in healthy volunteers as well as in patients with schizophrenia and Alzheimer’s disease (99). In addition, there is evidence indicating that centrally acting cholinergic drugs used in treatment of Alzheimer’s patients can modulate peripheral immune responses, which make this group of drugs interesting to explore in inflammatory disorders (100).
A novel antiinflammatory strategy also could be developed by means of optimal VNS generated by a special device. VNS has recently been shown to inhibit development of CIA in rats using a constant voltage stimulus (5 V, 2 ms, 1 Hz) starting at day 10 after the second immunization (101). Vagus nerve stimulation already is used in patients with drug-resistant epilepsy and depression. The left vagus nerve is stimulated via an implantable electrode. Vagus nerve stimulation had beneficial effects in both disorders without major side effects (99). A recent study investigating in more detail the effect of VNS on the immune system in 11 patients with refractory epilepsy demonstrated that VNS causes a rebalancing of the immune system compared with a control group (102). The effects of VNS on pro- and antiinflammatory cytokines in peripheral blood observed in this study in combination with the results found in the rat model of arthritis suggest that VNS could be a promising strategy in the treatment of RA patients.
Overall, data obtained from a large number of in vitro and in vivo studies imply that therapeutic agents targeting the PNS via the cholinergic antiinflammatory pathway or targeting the SNS via AR receptors can be an important future treatment option in a variety of conditions. However, further preclinical and clinical studies are needed to further explore the potential and safety of these approaches in patients with inflammatory disorders.
Disclosure
PP Tak recieved research support from SetPoint and Critical Therapeutics.
References
Bartok B, Firestein GS. (2010) Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233:233–55.
Tak PP, Bresnihan B. (2000) The pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis. Arthritis Rheum. 43:2619–33.
Guyton AC, Hall JE. (2000) Textbook of Medical Physiology. 10th edition. Philadelphia, PA: WB Saunders Company. 1064 pp.
Steinman L. (2004) Elaborate interactions between the immune and nervous systems. Nat. Immunol. 5:575–81.
Veale D, Farrell M, Fitzgerald O. (1993) Mechanism of joint sparing in a patient with unilateral psoriatic arthritis and a longstanding hemiplegia. Br. J. Rheumatol. 32:413–6.
Martin JH. (2003) Functional anatomy of autonomic nervous control. In: Neuroanatomy: Text and Atlas. McGraw-Hill Medical, New York, NY, pp. 358–63.
Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. (2000) The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 52:595–638.
Nance DM, Sanders VM. (2007) Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav. Immun. 21:736–45.
Miller LE, Justen HP, Scholmerich J, Straub RH. (2000) The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. FASEB J. 14:2097–107.
Bluthe RM, et al. (1994) Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C. R. Acad. Sci. III. 317:499–503.
Watkins LR, et al. (1995) Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci. Lett. 183:27–31.
Bellinger DL, et al. (2008) Sympathetic modulation of immunity: relevance to disease. Cell. Immunol. 252:27–56.
Lorton D, et al. (2009) Differences in the injury/sprouting response of splenic noradrenergic nerves in Lewis rats with adjuvant-induced arthritis compared with rats treated with 6-hydroxydopamine. Brain Behav. Immun. 23:276–85.
Straub RH, Rauch L, Fassold A, Lowin T, Pongratz G. (2008) Neuronally released sympathetic neurotransmitters stimulate splenic interferon-gamma secretion from T cells in early type II collagen-induced arthritis. Arthritis Rheum. 58:3450–60.
Hahn PY, Yoo P, Ba ZF, Chaudry IH, Wang P. (1998) Upregulation of Kupffer cell beta-adrenoceptors and cAMP levels during the late stage of sepsis. Biochim. Biophys. Acta. 1404:377–84.
Viswanathan K, Dhabhar FS. (2005) Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc. Natl. Acad. Sci. U. S. A. 102:5808–13.
Redwine L, Snow S, Mills P, Irwin M. (2003) Acute psychological stress: effects on chemotaxis and cellular adhesion molecule expression. Psychosom. Med. 65:598–603.
Goebel MU, Mills PJ. (2000) Acute psychological stress and exercise and changes in peripheral leukocyte adhesion molecule expression and density. Psychosom. Med. 62:664–70.
Evrengul H, et al. (2004) Heart rate variability in patients with rheumatoid arthritis. Rheumatol. Int. 24:198–202.
Straub RH, Dhabhar FS, Bijlsma JW, Cutolo M. (2005) How psychological stress via hormones and nerve fibers may exacerbate rheumatoid arthritis. Arthritis Rheum. 52:16–26.
Wilder RL. (1995) Neuroendocrine-immune system interactions and autoimmunity. Annu. Rev. Immunol. 13:307–38.
Coderre TJ, Basbaum AI, Dallman MF, Helms C, Levine JD. (1990) Epinephrine exacerbates arthritis by an action at presynaptic B2-adrenoceptors. Neuroscience. 34:521–3.
Levine JD, Coderre TJ, Helms C, Basbaum AI. (1988) Beta 2-adrenergic mechanisms in experimental arthritis. Proc. Natl. Acad. Sci. U. S. A. 85:4553–6.
Lubahn CL, Schaller JA, Bellinger DL, Sweeney S, Lorton D. (2004) The importance of timing of adrenergic drug delivery in relation to the induction and onset of adjuvant-induced arthritis. Brain Behav. Immun. 18:563–71.
Baerwald C, Graefe C, Muhl C, Von Wichert P, Krause A. (1992) Beta 2-adrenergic receptors on peripheral blood mononuclear cells in patients with rheumatic diseases. Eur. J. Clin. Invest. 22 Suppl 1:42–6.
Baerwald CG, et al. (1997) Impaired sympathetic influence on the immune response in patients with rheumatoid arthritis due to lymphocyte subset-specific modulation of beta 2-adrenergic receptors. Br. J. Rheumatol. 36:1262–9.
Wahle M, et al. (2005) Beta2-adrenergic receptors mediate the differential effects of catecholamines on cytokine production of PBMC. J. Interferon Cytokine Res. 25:384–94.
Kuis W, et al. (1996) The autonomic nervous system and the immune system in juvenile rheumatoid arthritis. Brain Behav. Immun. 10:387–98.
Rouppe van der Voort C, Kavelaars A, van de Pol M, Heijnen CJ. (1999) Neuroendocrine mediators up-regulate alpha1b- and alpha1d- adrenergic receptor subtypes in human monocytes. J. Neuroimmunol. 95:165–73.
Straub RH, Harle P. (2005) Sympathetic neurotransmitters in joint inflammation. Rheum. Dis. Clin. North. Am. 31:43–59, viii.
Wahle M, et al. (1999) Disease activity related catecholamine response of lymphocytes from patients with rheumatoid arthritis. Ann. N. Y. Acad. Sci. 876:287–96.
Heijnen CJ, et al. (1996) Functional alpha 1-adrenergic receptors on leukocytes of patients with polyarticular juvenile rheumatoid arthritis. J. Neuroimmunol. 71:223–6.
Coderre TJ, Basbaum AI, Helms C, Levine JD. (1991) High-dose epinephrine acts at alpha 2-adrenoceptors to suppress experimental arthritis. Brain Res. 544:325–8.
Malfait AM, et al. (1999) The beta2-adrenergic agonist salbutamol is a potent suppressor of established collagen-induced arthritis: mechanisms of action. J. Immunol. 162:6278–83.
Harle P, Mobius D, Carr DJ, Scholmerich J, Straub RH. (2005) An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheum. 52:1305–13.
Harle P, Pongratz G, Albrecht J, Tarner IH, Straub RH. (2008) An early sympathetic nervous system influence exacerbates collagen-induced arthritis via CD4+CD25+ cells. Arthritis Rheum. 58:2347–55.
Steiniger B, Barth P. (2000) Microanatomy and Function of the Spleen. New York: Spinger. Section 8.2, Blood Circulation in the Splenic Red Pulp: Subpopulations of Fibroblasts and Their Role; pp. 74–8. Advances in Anatomy, Embryology and Cell Biology; volume 151.
Lorton D, et al. (2005) Changes in the density and distribution of sympathetic nerves in spleens from Lewis rats with adjuvant-induced arthritis suggest that an injury and sprouting response occurs. J. Comp. Neurol. 489:260–73.
del Rey A, et al. (2008) Disrupted brain-immune system-joint communication during experimental arthritis. Arthritis Rheum. 58:3090–9.
Miller LE, Grifka J, Scholmerich J, Straub RH. (2002) Norepinephrine from synovial tyrosine hydroxylase positive cells is a strong indicator of synovial inflammation in rheumatoid arthritis. J. Rheumatol. 29:427–35.
Capellino S, et al. (2010) Catecholamine-producing cells in the synovial tissue during arthritis: modulation of sympathetic neurotransmitters as new therapeutic target. Ann. Rheum. Dis. 69:1853–60.
Levine JD, et al. (1984) Intraneuronal substance P contributes to the severity of experimental arthritis. Science. 226:547–9.
Berthoud HR, Neuhuber WL. (2000) Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 85:1–17.
Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. (2003) The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol. Med. 9:125–34.
de Jonge WJ, et al. (2005) Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 6:844–51.
Kalamida D, et al. (2007) Muscle and neuronal nicotinic acetylcholine receptors. Structure, function and pathogenicity. FEBS J. 274:3799–845.
Wess J. (1996) Molecular biology of muscarinic acetylcholine receptors. Crit. Rev. Neurobiol. 10:69–99.
Wessler I, Kilbinger H, Bittinger F, Unger R, Kirkpatrick CJ. (2003) The non-neuronal cholinergic system in humans: expression, function and pathophysiology. Life Sci. 72:2055–61.
Wessler I, Kirkpatrick CJ. (2008) Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br. J. Pharmacol. 154:1558–71.
Wess J. (1996) Molecular biology of muscarinic acetylcholine receptors. Crit. Rev. Neurobiol. 10:69–99.
Buijs RM, van der Vliet J, Garidou ML, Huitinga I, Escobar C. (2008) Spleen vagal denervation inhibits the production of antibodies to circulating antigens. PLoS One. 3:e3152.
Rosas-Ballina M, et al. (2008) Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. U. S. A. 105:11008–13.
Gaykema RP, Dijkstra I, Tilders FJ. (1995) Subdi-aphragmatic vagotomy suppresses endotoxin-induced activation of hypothalamic corticotropin-releasing hormone neurons and ACTH secretion. Endocrinology. 136:4717–20.
Gaykema RP, Chen CC, Goehler LE. (2007) Organization of immune-responsive medullary projections to the bed nucleus of the stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus: evidence for parallel viscerosensory pathways in the rat brain. Brain Res. 1130:130–45.
Goehler LE, et al. (1997) Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res. Bull. 43:357–64.
Hosoi T, Okuma Y, Nomura Y. (2000) Electrical stimulation of afferent vagus nerve induces IL-1beta expression in the brain and activates HPA axis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279:R141–7.
Maier SF, Goehler LE, Fleshner M, Watkins LR. (1998) The role of the vagus nerve in cytokine-to-brain communication. Ann. N. Y. Acad. Sci. 840:289–300.
Borovikova LV, et al. (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 405:458–62.
Bernik TR, et al. (2002) Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J. Vasc. Surg. 36:1231–6.
Guarini S, et al. (2003) Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 107:1189–94.
Saeed RW, et al. (2005) Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J. Exp. Med. 201:1113–23.
Tracey KJ. (2009) Reflex control of immunity. Nat. Rev. Immunol. 9:418–28.
Kalamida D, et al. (2007) Muscle and neuronal nicotinic acetylcholine receptors. Structure, function and pathogenicity. FEBS J. 274:3799–845.
Wang H, et al. (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 421:384–8.
Kox M, et al. (2009) GTS-21 inhibits pro-inflammatory cytokine release independent of the Toll-like receptor stimulated via a transcriptional mechanism involving JAK2 activation. Biochem. Pharmacol. 78:863–72.
Rosas-Ballina M, et al. (2009) The selective alpha7 agonist GTS-21 attenuates cytokine production in 948 human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol. Med. 15:195–202.
Drisdel RC, Green WN. (2000) Neuronal alpha-bungarotoxin receptors are alpha7 subunit homomers. J. Neurosci. 20:133–9.
de Jonge WJ, Ulloa L. (2007) The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br. J. Pharmacol. 151:915–29.
van Maanen MA, et al. (2009) The alpha7 nicotinic acetylcholine receptor on fibroblast-like synoviocytes and in synovial tissue from rheumatoid arthritis patients: a possible role for a key neurotransmitter in synovial inflammation. Arthritis Rheum. 60:1272–81.
Waldburger JM, Boyle DL, Pavlov VA, Tracey KJ, Firestein GS. (2008) Acetylcholine regulation of synoviocyte cytokine expression by the alpha7 nicotinic receptor. Arthritis Rheum. 58:3439–49.
Westman M, Engstrom M, Catrina AI, Lampa J. (2009) Cell specific synovial expression of nicotinic alpha 7 acetylcholine receptor in rheumatoid arthritis and psoriatic arthritis. Scand. J. Immunol. 70:136–40.
Villiger Y, et al. (2002) Expression of an alpha7 duplicate nicotinic acetylcholine receptor-related protein in human leukocytes. J. Neuroimmunol. 126:86–98.
Giebelen IA, van Westerloo DJ, LaRosa GJ, de Vos AF, van der Poll T. (2007) Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock. 28:700–3.
Pavlov VA, et al. (2007) Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit. Care Med. 35:1139–44.
van Westerloo DJ, et al. (2006) The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 130:1822–30.
Yeboah MM, et al. (2008) Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 74:62–9.
The FO, et al. (2007) Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology. 133:1219–28.
van Maanen MA, et al. (2009) Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 60:114–22.
Li T, et al. (2010) The vagus nerve and nicotinic receptors involve inhibition of HMGB1 release and early pro-inflammatory cytokines function in collagen-induced arthritis. J. Clin. Immunol. 30:213–20.
Huston JM, et al. (2006) Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 203:1623–8.
Spengler RN, Chensue SW, Giacherio DA, Blenk N, Kunkel SL. (1994) Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J. Immunol. 152:3024–31.
Kees MG, Pongratz G, Kees F, Scholmerich J, Straub RH. (2003) Via beta-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced TNF secretion in perfused rat spleen. J. Neuroimmunol. 145:77–85.
Brandon KW, Rand MJ. (1961) Acetylcholine and the sympathetic innervation of the spleen. J. Physiol. 157:18–32.
Rinner I, Kawashima K, Schauenstein K. (1998) Rat lymphocytes produce and secrete acetylcholine in dependence of differentiation and activation. J. Neuroimmunol. 81:31–7.
Cai B, et al. (2009) Alpha7 cholinergic-agonist prevents systemic inflammation and improves survival during resuscitation. J. Cell. Mol. Med. 13:3774–85.
van Maanen MA, Stoof SP, LaRosa GJ, Vervoordeldonk MJ, Tak PP. (2010) Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor alpha7 subunit gene knockout mice. Ann. Rheum. Dis. 69:1717–23.
Bruchfeld A, et al. (2010) Whole blood cytokine attenuation by cholinergic agonists ex vivo and relationship to vagus nerve activity in rheumatoid arthritis. J. Intern. Med. 268:94–101.
Grimsholm O, Rantapaa-Dahlqvist S, Dalen T, Forsgren S. (2008) Unexpected finding of a marked non-neuronal cholinergic system in human knee joint synovial tissue. Neurosci. Lett. 442:128–33.
Forsgren S, Grimsholm O, Jonsson M, Alfredson H, Danielson P. (2009) New insight into the nonneuronal cholinergic system via studies on chronically painful tendons and inflammatory situations. Life Sci. 84:865–70.
Moriwaki Y, et al. (2007) Immune system expression of SLURP-1 and SLURP-2, two endogenous nicotinic acetylcholine receptor ligands. Life Sci. 80:2365–8.
Dekkers JC, Geenen R, Godaert GL, Bijlsma JW, van Doornen LJ. (2004) Elevated sympathetic nervous system activity in patients with recently diagnosed rheumatoid arthritis with active disease. Clin. Exp. Rheumatol. 22:63–70.
Goldstein RS, et al. (2007) Cholinergic antiinflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol. Med. 13:210–5.
Harle P, et al. (2006) Increase of sympathetic outflow measured by neuropeptide Y and decrease of the hypothalamic-pituitary-adrenal axis tone in patients with systemic lupus erythematosus and rheumatoid arthritis: another example of uncoupling of response systems. Ann. Rheum. Dis. 65:51–6.
Louthrenoo W, Ruttanaumpawan P, Aramrattana A, Sukitawut W. (1999) Cardiovascular autonomic nervous system dysfunction in patients with rheumatoid arthritis and systemic lupus erythematosus. QJM. 92:97–102.
Stojanovich L, et al. (2007) Cardiovascular autonomic dysfunction in systemic lupus, rheumatoid arthritis, primary Sjogren syndrome and other autoimmune diseases. Lupus. 16:181–5.
Straub RH, Paimela L, Peltomaa R, Scholmerich J, Leirisalo-Repo M. (2002) Inadequately low serum levels of steroid hormones in relation to interleukin-6 and tumor necrosis factor in untreated patients with early rheumatoid arthritis and reactive arthritis. Arthritis Rheum. 46:654–62.
Carlens C, Brandt L, Klareskog L, Lampa J, Askling J. (2007) The inflammatory reflex and risk for rheumatoid arthritis: a case-control study of human vagotomy. Ann. Rheum. Dis. 66:414–6.
van der Zanden EP, Boeckxstaens GE, de Jonge WJ. (2009) The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol. Motil. 21:6–17.
Shafique S, Dalsing MC. (2006) Vagus nerve stimulation therapy for treatment of drug-resistant epilepsy and depression. Perspect. Vasc. Surg. Endovasc. Ther. 18:323–7.
Pavlov VA, et al. (2006) Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl. Acad. Sci. U. S. A. 103:5219–23.
Zhang P, Han D, Tang T, Zhang X, Dai K. (2008) Inhibition of the development of collagen-induced arthritis in Wistar rats through vagus nerve suspension: a 3-month observation. Inflamm. Res. 57:322–8.
Majoie HJ, et al. (2011) Vagus nerve stimulation in refractory epilepsy: effects on pro- and antiinflammatory cytokines in peripheral blood. Neuroimmunomodulation. 18:52–6.
Acknowledgments
Supported by Dutch Arthritis Association grant NR 09-1-307. In addition, the authors would like to thank Beatrijs M Lodde for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Koopman, F.A., Stoof, S.P., Straub, R.H. et al. Restoring the Balance of the Autonomic Nervous System as an Innovative Approach to the Treatment of Rheumatoid Arthritis. Mol Med 17, 937–948 (2011). https://doi.org/10.2119/molmed.2011.00065
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2011.00065