Abstract
The German Multicenter EPO Stroke Trial, which investigated safety and efficacy of erythropoietin (EPO) treatment in ischemic stroke, was formally declared a negative study. Exploratory subgroup analysis, however, revealed that patients not receiving thrombolysis most likely benefited from EPO during clinical recovery, a result demonstrated in the findings of the Göttingen EPO Stroke Study. The present work investigated whether the positive signal on clinical outcome in this patient subgroup was mirrored by respective poststroke biomarker profiles. All patients of the German Multicenter EPO Stroke Trial nonqualifying for thrombolysis were included if they (a) were treated per protocol and (b) had at least two of the five follow-up blood samples for circulating damage markers drawn (n = 163). The glial markers S100B and glial fibrillary acid protein (GFAP) and the neuronal marker ubiquitin C-terminal hydrolase (UCH-L1) were measured by enzyme-linked immunosorbent assay in serum on d 1, 2, 3, 4 and 7 poststroke. All biomarkers increased poststroke. Overall, EPO-treated patients had significantly lower concentrations (area under the curve) over 7 d of observation, as reflected by the composite score of all three markers (Cronbach α = 0.811) and by UCH-L1. S100B and GFAP showed a similar tendency. To conclude, serum biomarker profiles, as an outcome measure of brain damage, corroborate an advantageous effect of EPO in ischemic stroke. In particular, reduction in the neuronal damage marker UCH-L1 may reflect neuroprotection by EPO.
Similar content being viewed by others
Introduction
Since 1998, nearly 200 preclinical studies have proven erythropoietin (EPO) to have neuroprotective and neuroregenerative potential, ranging from ischemia and neurotrauma to inflammation and neurodegeneration. Essentially all of the few clinical studies performed in the neuroscience field have yielded positive results with respect to EPO treatment effects (reviewed in [1,2]). The first encouraging clinical study was the Göttingen EPO Stroke Study, which showed beneficial outcome of ischemic stroke patients upon EPO (3).
Unfortunately, the respective follow-up study, the German Multicenter EPO Stroke Trial (ClinicalTrials.gov identifier NCT00604630), building on these positive results, turned out to be a formally negative trial, because of the unexpectedly large percentage of recombinant tissue plasminogen activator (rtPA) treatments with a high violation rate of thrombolysis contraindications (4). Patients with rtPA application despite prior anticoagulation had inferior outcome under additional EPO, whereas patients treated with rtPA “lege artis” (“correctly”; “by law of art”) did not have any disadvantage of EPO treatment (https://doi.org/www.epo-study.de) (4). Potential mechanisms explaining a negative interplay between EPO and rtPA were recently reported in preclinical work (5,6). In contrast, patients who did not receive rtPA likely benefitted from EPO with a clinical course/outcome (National Institutes of Health Stroke Scale [NIHSS]) comparable to that obtained in the first EPO stroke study (3,4). In the absence of any other neuroprotective or neuroregenerative strategy available for stroke patients, this promising signal encourages further work along these lines.
Circulating biomarkers of brain damage are increasingly considered as additional outcome measures for stroke complementing clinical and imaging data. Among the markers selected for the present analysis, the glial damage markers S100B and glial fibrillary acidic protein (GFAP) have been in clinical use for many years, whereas the neuronal marker ubiquitin C-terminal hydrolase (UCH-L1) has been integrated recently in the repertoire of stroke biomarkers. All these damage markers correlate well with clinical severity, course and outcome of brain injury (7,8).
UCH-L1 is a highly abundant protein that resides in almost all neurons and averages between 1% and 5% of total soluble brain proteins. It has been suggested that UCH-L1 plays a critical role in the removal of excessively oxidized or misfolded proteins both during normal and neuropathological conditions (9–12). On the basis of this important neuronal function and its high specificity and abundance in the central nervous system, we selected UCH-L1 here as a candidate biomarker for poststroke brain injury and readout of neuroprotection.
S100B is a low-molecular-weight glial protein of a multigenic family of calcium-binding proteins, highly specific to the nervous system and found in abundance in the astroglia compartment in the cerebral cortex, in peripheral Schwann cells, but also extra-neuronally in melanocytes, adipocytes and chondrocytes (13). In previous studies, we could show that S100B release was associated with stroke severity and clinical outcome (14). S100B was also postulated to be a marker of generalized blood-brain barrier dysfunction, rather than of specific glia damage only (15).
GFAP is a monomeric filament protein localized to astrocytes in the brain. GFAP is involved in various neuronal processes, including maintenance of the blood-brain barrier (reviewed in [16]). Increased serum concentrations of GFAP were described after ischemic stroke and traumatic brain injury and to correlate with clinical severity and outcome (17,18).
About 10 years ago, we argued that molecular markers of brain damage might be a useful tool in translational stroke research and that the analysis of the release patterns of biomarkers might be a promising strategy to evaluate neuroprotective approaches in stroke treatment (19). Here we report poststroke biomarker profiles of an exploratory subgroup comprising per-protocol treated ischemic stroke patients of the German Multicenter EPO Stroke Trial who did not receive rtPA.
Materials and Methods
Patients
The present predefined exploratory subgroup analysis is based on all patients of the randomized, double-blind, placebo-controlled German Multicenter EPO Stroke Trial (4) who fulfilled the following requirements: (a) they were treated per-protocol, (b) they had not received rtPA, and (c) they had at least two out of five follow-up blood samples for circulating damage markers drawn, resulting in a total of n = 163 patients (exclusion of n = 3 due to missing serum samples). Main inclusion criteria were acute ischemic stroke in the middle cerebral artery territory leading to a score ≥4 in the NIHSS.
Study Intervention
Intravenous infusion of recombinant human EPO (Epoetin-α, 40,000 IU) or placebo was given within 6 h after symptom onset (day 1) and repeated 24 and 48 h later (4). The dose was chosen according to the previous EPO study (3).
Biomarker Assays
Blood for biomarker analysis was drawn on d 1, 2, 3, 4 and 7. Serum was stored at −80°C. Enzyme-linked immunosorbent assays of S100B, GFAP and UCH-L1 were performed blindly (that is, without clinical information) using antibodies from Banyan (Alachua, FL, USA), Sigma (St. Louis, MO, USA) and Dako (Carpentaria, CA, USA).
Statistical Analysis
For each marker, a linear regression-based multiple imputation (10 iterations) model of missing data (UCH-L1 5.8%; S100B 6.5%; GFAP 20.6% missing) was applied, if at least two out of five values per subject were present (resulting in n = 163 subjects for UCH-L1 and S100B and n = 154 for GFAP). All per-protocol treated non-rtPA individuals not meeting this criterion were excluded from further analysis (UCH-L1 and S100B n = 3; GFAP n = 12). Areas under the curve (AUCs) for every marker were determined for each imputation matrix by the composite trapezoidal rule for numerical integration. The pooled AUC represents the mean of the 10 AUC matrices per marker. Two composite scores were calculated reflecting the mean of the z-standardized pooled AUC values for UCH-L1, S100B and GFAP (Cron-bach α = 0.811) and for S100B and GFAP (Cronbach α = 0.755). For a total of n = 9 individuals, the composite scores had to be based on the z-standardized pooled AUC values for UCH-L1 and S100B only. Mann-Whitney U tests (two-tailed) and χ2 tests or Fisher exact test were used for intergroup comparisons. Analysis of variance for repeated measures was applied to compare EPO versus placebo with respect to Δ NIHSS (NIHSS at baseline — NIHSS day 90). Analysis of covariance with NIHSS score at baseline as the covariate compared both groups with respect to pooled single-marker AUC values and AUC composite scores. Further, a correlation analysis (Pearson) of Δ NIHSS and UCH-L1 AUC was performed. Data are presented as mean ± SD in text/tables and median or mean ± SEM in figures.
Results
Patient characteristics at inclusion were well balanced between EPO and placebo groups in all important baseline variables, representative of a typical stroke population (Table 1). Biomarker profiles in serum displayed the expected increases between d 2 and 4 poststroke, with peak time points varying considerably among different markers and individual patients (Figure 1).
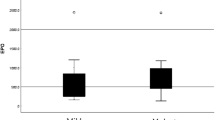
The clinical course of included per-protocol treated non-rtPA patients (n = 163) demonstrates a slightly better outcome of the EPO compared with the placebo group (mean Δ NIHSS of 5.3 ± 5.3 in EPO versus 3.3 ± 6.5 in placebo; P = 0.039) (Figure 2A). As a best estimate of the total increase in circulating damage marker concentrations, AUCs were calculated for each marker in all patients. AUCs, corrected for NIHSS d 1 (severity of stroke symptoms upon inclusion, that is, before any study drug treatment), turned out to be significantly lower in EPO versus placebo patients for UCH-L1 and showed a similar tendency for S100B and GFAP (Figure 2B).
Biomarkers substantiate the positive signal on clinical outcome of EPO compared with placebo patients. (A) EPO patients show improved clinical outcome (NIHSS) after stroke compared with the placebo group. AUC mean ± SEM values (B) and AUC z-standardized values (C) demonstrate differences in biomarkers poststroke between EPO and placebo patients. (D) Δ NIHSS and UCH-L1 AUC correlate in both treatment groups with a numerically higher correlation coefficient in EPO patients.
To make use of the complete bio-marker information, a z-standardization of the AUC scores for each marker was performed. AUC composite scores of the two glial markers and of all three bio-markers were calculated. The internal consistency of these composite scores turned out to be sufficiently high (n = 154; Cronbach α = 0.755; and n = 154; Cronbach α = 0.811, respectively) to justify their use as composites. Figure 2C illustrates z-standardized biomarker AUC levels for all single markers and the two composites showing that all three biomarkers discriminate between EPO and placebo groups, with UCH-L1 as a single marker and the three-marker composite score reaching statistical significance. Correlation coefficients of Δ NIHSS and UCH-L1 AUC were found to be significant for both treatment groups, with a numerically higher value in EPO patients (Figure 2D). This result again emphasizes the neuroprotective property of EPO in stroke.
Discussion
The present exploratory subgroup study builds on the observation that stroke patients of the German Multicenter EPO Stroke Trial, nonqualifying for rtPA treatment, seemed to have a better clinical course/outcome under EPO than placebo (4). This observation is further supported here by respective stroke bio-marker profiles. The blunted increase in the neuronal damage marker UCH-L1 under EPO especially points to neuroprotection. The results obtained with this marker may actually suggest its extended use as a surrogate marker of stroke severity in future neuroprotection trials.
Similar to the first EPO stroke study (3), the S100B increase tended to be lower in EPO patients but failed to reach statistical significance here. The third evaluated marker, GFAP, turned out to be the least responsive one in the present analysis, perhaps due to the necessity (insufficient sample volume left) to impute circa 20% of missing data (as compared with only around 6% for S100B and UCH-L1). The composite score of both glial markers, S100B and GFAP, produced a “near-significant” result. The composite of all three markers, although different between treatment groups, does at first look not add to the information obtainable with the neuronal marker UCH-L1 alone. However, both composite scores reveal that all contributing markers, be it of glial or neuronal origin, essentially behave synergistically.
In conclusion, stroke is a very common, devastating and frequently severely disabling condition with only thrombolysis and supportive measures presently available for treatment. The former still reaches just a small percentage of patients, and the increasing violation of rtPA contraindications (as also experienced in the German Multicenter EPO Stroke Trial) reflects desperation and fatalism of treating personnel in the absence of alternative therapeutic options. Importantly, stroke patients are extremely heterogeneous with respect to genetic and environmental predisposing factors, including comorbidities, explaining why huge effects of novel treatment strategies can never be expected over all patients. Therefore, even the slightest signal of benefit of neuroprotective treatment strategies has to be vigorously pursued. In this regard, the course of circulating brain damage markers upon EPO (in association with the documented clinical improvement) should encourage further work on EPO or EPO variants/analogs in ischemic stroke patients who are not eligible for thrombolysis.
Disclosure
H Ehrenreich holds/has submitted patents on EPO for stroke, schizophrenia and multiple sclerosis. J Streeter, KK Wang, RL Hayes and A Jeromin are employees of Banyan Biomarkers, Inc., a company developing biomarkers for brain diseases. All other authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Brines M, Cerami A. (2005) Emerging biological roles for erythropoietin in the nervous system. Nat. Rev. Neurosci. 6:484–94.
Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H. (2010) Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best Pract. Res. Clin. Anaestesiol. 24:573–94.
Ehrenreich H, et al. (2002) Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med. 8:495–505.
Ehrenreich H, et al. (2009) Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 40:e647–56.
Jia L, Chopp M, Zhang L, Lu M, Zhang Z. (2010) Erythropoietin in combination of tissue plasminogen activator exacerbates brain hemorrhage when treatment is initiated 6 hours after stroke. Stroke. 41:2071–6.
Zechariah A, ElAli A, Hermann DM. (2010) Combination of tissue-plasminogen activator with erythropoietin induces blood-brain barrier permeability, extracellular matrix disaggregation, and DNA fragmentation after focal cerebral ischemia in mice. Stroke. 41:1008–12.
Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ. (2000) Release of glial tissue-specific proteins after acute stroke: a comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 31:2670–7.
Papa L, et al. (2010) Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit. Care Med. 38:138–44.
Lewis SB, et al. (2010) Identification and preliminary characterization of ubiquitin C terminal hydrolase 1 (UCHL1) as a biomarker of neuronal loss in aneurysmal subarachnoid hemorrhage. J. Neurosci. Res. 88:1475–84.
Liu MC, et al. (2010) Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur. J. Neurosci. 31:722–32.
Siman R, et al. (2008) Biomarker evidence for mild central nervous system injury after surgically-induced circulation arrest. Brain Res. 1213:1–11.
Siman R, et al. (2009) A panel of neuron-enriched proteins as markers for traumatic brain injury in humans. J. Neurotrauma. 26:1867–77.
Heizmann CW. (1999) Ca2+-binding S100 proteins in the central nervous system. Neurochem. Res. 24:1097–100.
Wunderlich MT, et al. (1999) Early neurobehavioral outcome after stroke is related to release of neurobiochemical markers of brain damage. Stroke. 30:1190–5.
Marchi N, et al. (2003) Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor. Neurol. Neurosci. 21:109–21.
Eng LF, Ghirnikar RS, Lee YL. (2000) Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 25:1439–51.
Herrmann M, et al. (2000) Neurobehavioral outcome prediction after cardiac surgery: role of neurobiochemical markers of damage to neuronal and glial brain tissue. Stroke. 31:645–50.
Pelinka LE, et al. (2004) GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J. Neurotrauma. 21:1553–61.
Herrmann M, Ehrenreich H. (2003) Brain derived proteins as markers of acute stroke: their relation to pathophysiology, outcome prediction and neuroprotective drug monitoring. Restor. Neurol. Neurosci. 21:177–90.
Acknowledgments
This study was supported by the Max Planck Society and Banyan Biomarkers, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ehrenreich, H., Kästner, A., Weissenborn, K. et al. Circulating Damage Marker Profiles Support a Neuroprotective Effect of Erythropoietin in Ischemic Stroke Patients. Mol Med 17, 1306–1310 (2011). https://doi.org/10.2119/molmed.2011.00259
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2011.00259