Abstract
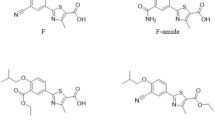
A micellar electrokinetic chromatography (MEKC) method was validated for the analysis of ezetimibe. The method was carried out on a fused-silica capillary (50 pm i.d.; effective length, 40 cm). The background electrolyte consisted of a 25 mM borate buffer and 25 mM anionic detergent SDS (pH 9.75)/methanol (90:10, v/v). The capillary temperature was maintained at 35°C, the applied voltage was 30 kV; the injection was performed using a pressure mode at 50 mbar for 5 s, with detection at 232 nm. The method was linear in the range of 2–150 pg/mL (R2 = 0.9999). The specificity and the stability-indicating capability were proven through degradation studies, which also showed that there was no interference of the excipients. The limits of quantitation and detection were 2 and 0.41 pg/mL, respectively. The method was applied for the analysis of ezetimibe pharmaceutical formulations, and the results were compared to those of the liquid- chromatography method.
Similar content being viewed by others
References
A. L. Catapano, Eur. Heart J. Suppl., 2001, 3(Suppl. E), E6.
J. W. Clader, J. Med. Chem., 2004, 47, 1.
S. W. Altmann, H. R. Davis, Jr., L. Zhu, X. Yao, L. M. Hoos, G. Tetzloff, S. P. N. Iyer, M. Maguire, A. Golovko, M. Zeng, L. Wang, N. Murgolo, and M. P. Graziano, Science, 2004, 303, 1201.
J. J. P. Kastelein, P. T. Sager, E. de Groot, and E. Veltri, Am. Heart J., 2005, 149, 234.
C. M. Ballantyne, N. Abate, Z. Yuan, T. R. King, and J. Palmisano, Am. Heart J., 2005, 149, 464.
C. M. Ballantyne, R. Weiss, T. Moccetti, A. Vogt, B. Eber, F. Sosef, and E. Duffield, Am. J. Cardiol., 2007, 99, 673.
R. Sistla, V. S. S. K. Tata, Y. V. Kashyap, D. Chandrasekar, and P. V. Diwan, J. Pharm. Biomed. Anal., 2005, 39, 517.
S. Singh, B. Singh, R. Bahuguna, L. Wadhwa, and R. Saxena, J. Pharm. Biomed. Anal., 2006, 41, 1037.
S. Li, G. Liu, J. Jia, X. Li, and C. Yu, J. Pharm. Biomed. Anal., 2006, 40, 987.
P. R. Oliveira, L. Brum, Jr., M. Fronza, L. S. Bernardi, S. M. K. Masiero, and S. L. Dalmora, Chromatographia, 2006, 63, 315.
S. Oswald, E. Scheuch, I. Cascorbi, and W. Siegmund, J. Chromatogr., B, 2006, 830, 143.
K. D. Altria and D. Elder, J. Chromatogr., A, 2004, 1023, 1.
L. A. Holland, N. P. Chetwyn, M. D. Perkins, and S. M. Lunte, Pharm. Res., 1997, 14, 372.
T. G. Morzunova, Pharm. Chem. J., 2006, 40, 158.
U. Holzgrabe, D. Brinz, S. Kopec, C. Weber, and Y. Bitar, Electrophoresis, 2006, 27, 2283.
P. R. Oliveira, T. Barth, V. Todeschini, and S. L. Dalmora, J. AOAC Int., in press.
ICH, “Validation of Analytical Procedures: Text and Methodology Q2(R1)”, in Proceedings of the International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use, http://www.ich.org.
ICH, “Stability Testing of New Drugs Substance and Products Q1A(R2)”, in Proceedings of the International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use, http://www.ich.org.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dalmora, S.L., Oliveira, P.R., Barth, T. et al. Development and Validation of a Stability-indicating Micellar Electrokinetic Chromatography Method for the Determination of Ezetimibe in Pharmaceutical Formulations. ANAL. SCI. 24, 499–503 (2008). https://doi.org/10.2116/analsci.24.499
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.24.499