Abstract
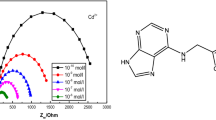
A poly(vinyl chloride)-based membrane of 3,4:11,12-dibenzo-1,6,9,14-tetraazacyclohexadecane with sodium tetraphenyl borate (NaTPB) as an anion excluder and dibutylphthalate (DBP), dibutyl(butyl)phosphonate (DBBP), tris(2-ethylhexyl)phosphate (TEHP) and tributyl phosphate (TBP) as a plasticizing solvent mediator was prepared and investigated as a Cd2+-selective electrode. The best performance was observed with the membrane having a ligand-PVC-DBP-NaTPB composition of 2:25:60:1, which worked well over a wide concentration range (1.6 × 10–6–1.0 × 10–1 M) with a Nernstian slope of 29.5 mV per decade of activity between pH 2.0–6.0. This electrode showed a fast response time of 13 s and was used over a period of 4 months with good reproducibility (S = 0.4 mV). The selectivity coefficient for mono-, di- and trivalent cations indicated excellent selectivity for Cd2+ ions over a large number of cations. Anions such as NO3– and SO42–, did not interfere. The membrane sensor has been successfully used to determine Cd2+ in real samples.
Similar content being viewed by others
References
R. Henry and H. Eugene, “Biological Chemistry”, 1971, Harper and Row, New York, 686.
A. K. De, “Environmental Chemistry”, 1994, New Age International, New Delhi, India, 80.
A. C. Slevens and H. Freiser, Anal. Chim. Acta, 1991, 241, 315.
P. L. H. M. Cobben, R. J. M. Egberink, J. B. Bomer, P. Bergveld, W. Verboom, and D. D. Reinhoudt, J. Am. Chem. Soc, 1992, 114, 10573.
S. K. Srivastava, V. K. Gupta, and S. Jain, Electroanalysis, 1996, 8, 938.
V. Asano and H. Wada, Talanta, 1997, 44, 697.
V. K. Gupta, P. Kumar, and R. Mangla, Electroanalysis, 2000, 12, 10.
V. K. Gupta, S. Chandra, and R. Mangla, Electrochim. Acta, 2002, 47, 1579.
A. Panwar, S. Baniwal, C. L. Sharma, and A. K. Singh, Fresenius J. Anal. Chem., 2000, 368, 768.
M. Shakir and S. P. Varkey, Ind. J. Chem., 1995, 34A, 355.
R. P. Buck and V. V. Cosofret, Pure Appl. Chem., 1993, 65, 1849.
U. Oesch and W. Simon, Anal. Chem., 1980, 52, 692.
E. Bakker, E. Pretsch, and P. Bühlmann, Anal. Chem., 2000, 72, 1127.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, K., Saxena, P. & Singh, R. New Cadmium(II)-Selective Electrode Based on a Tetraazacyclohexadeca Macrocyclic Ionophore Ashok. ANAL. SCI. 21, 179–181 (2005). https://doi.org/10.2116/analsci.21.179
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.21.179