Abstract
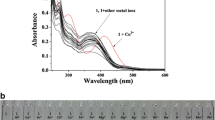
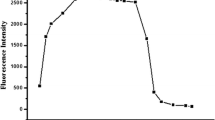
A colorimetric and turn-on fluorescent chemodosimeter 1 based on diaminomaleonitrile was synthesized for Cu2+ detection. It showed high selectivity and sensitivity towards Cu2+ over the other tested metal ions. Probe 1 in acetonitrile exhibited a strong absorption band at 530 nm and weak fluorescence emission when excited at 480 nm, while the addition of Cu2+ could lead to a 30-nm blue shift of the absorption band and a remarkable fluorescence enhancement. Moreover, the detection limit of probe 1 for Cu2+ was calculated to be 28 nM. Quite different from the reported mechanism based on a metal-complexation induced fluorescence enhancement, the sensing mechanism was proved to be based on the Cu2+-promoted hydrolysis reaction, which was confirmed by 1H NMR, 13C NMR and mass spectrum analysis. Studies on probe 2 were carried out to verify the universality of this sensing mechanism.
Similar content being viewed by others
References
B. R. Stem, J. Toxicol. Environ. Healt, Part A, 2010, 73, 114.
E. Gaggelli, H. Kozlowski, D. Valensin, and G. Valensin, Chem. Rev., 2006, 106, 1995.
P. G. Georgopoulos, A. Roy, M. J. Yonone-Lioy, R. E. Opiekun, and P. J. Lioy, J. Toxicol. Environ. Health, Part B, 2001, 4, 341.
R. B. An, D. T. Zhang, Y. Chen, and Y. Z. Cui, Sens. Actuators, B, 2016, 222, 48.
H. Yu, J. Y. Lee, S. Angupillai, S. Wang, S. H. Feng, S. Matsumoto, and Y. A. Son, Spectrochim. Acta, Part A, 2015, 151, 48.
X. F Meng, Y. X. Xu, J. L. Liu, L. N. Sun, and L. Y. Shi, Anal. Methods, 2016, 8, 1044.
M. S. Kim, J. M. Jung, J. H. Kang, H. M. Ahn, P G. Kim, and C. Kim, Tetrahedron, 2017, 73, 4570.
D. Udhayakumari, S. Naha, and S. Velmathi, Anal. Methods, 2017, 9, 552.
G. Sivaraman, M. Iniya, T. Anand, N. G. Kotla, O. Sunnapu, S. Singaravadivel, A. Gulyani, and D. Chellappa, Coord. Chem. Rev., 2018, 357, 50.
B. Kaur, N. Kaur, and S. Kumar, Coord. Chem. Rev., 2018, 358, 13.
T. Sun, Q. F Niu, T. D. Li, Z. R. Guo, and H. X. Liu, Spectrochim. Acta, Part A, 2018, 188, 411.
W. T. Li, G. H. Zhu, J. H. Li, Z. Q. Wang, and Y. X. Jin, Molecules, 2016, 21, 107.
L. Q. Li, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2016, 46, 1854.
H. Y. Liu, F. X. Wu, B. B. Zhang, C. Y. Tan, Y. Z. Chen, G. F Hao, Y. Tan, and Y. Y. Jiang, RSC Adv., 2016, 6, 7750.
C. M. Li, Z. X. Liu, Y. Miao, X. Zhou, and X. Wu, Dyes Pigm., 2016, 125, 292.
F. Zhao, Y. Hu, Q. Li, and S. L. Hu, Heterocycles, 2017, 94, 515.
P. ReddyPrasad and T Imae, Taiwan Inst. Chem. E, 2017, 72, 194.
M. J. Wei, Y. Y. Zhang, H. T Li, and S. Z. Yao, Anal. Methods, 2017, 9, 3956.
Y. P. Wang, D. L. Qiu, M. N. Li, Y. J. Liu, H. B. Chen, and H. M. Li, Spectrochim. Acta, Part A, 2017, 185, 256.
K. Mahesh and S. Karpagam, Sens. Actuators, B, 2017, 251, 9.
J. Xu, Z. K. Wang, C. Y. Liu, Z. H. Xu, B. C. Zhu, N. Wang, K. Wang, and J. T. Wang, Anal. Sci., 2018, 34, 453.
Y. Zeng, G. X. Zhang, and D. Q. Zhang, Anal. Sci., 2015, 31, 191.
R. Kato, Anal. Sci., 2018, 34, 395.
S. P Wu, T. H. Wang, and S. R. Liu, Tetrahedron, 2010, 66, 9655.
H. C. Lan, B. Liu, G. L. Lv, Z. H. Li, X. D. Yu, K. Y. Liu, X. H. Cao, H. Yang, S. P. Yang, and T. Yi, Sens. Actuators, B, 2012, 173, 811.
H. P. Zhou, J. Q. Wang, Y. X. Chen, W. G. Xi, Z. Zheng, D. L. Xu, Y. L. Cao, G. Lin, W. J. Zhu, J. Y. Wu, and Y. P Tian, Dyes Pigm., 2013, 98, 1.
W. G. Xia, Y. M. Gong, B. Mei, X. Z. Zhang, Y. B. Zhang, B. Y. Chen, J. Y. Wu, Y. P. Tian, and H. P. Zhou, Sens. Actuators, B, 2014, 205, 158.
T. G. Jo, Y. J. Na, J. J. Lee, M. M. Lee, S. Y. Lee, and C. Kim, New J. Chem., 2015, 39, 2580.
T. G. Jo, Y. J. Na, J. J. Lee, M. M. Lee, S. Y. Lee, and C. Kim, Sens. Actuators, B, 2015, 211, 498.
S. A. Lee, J. J. Lee, J. W. Shin, K. S. Min, and C. Kim, Dyes Pigm., 2015, 116, 131.
I. J. Chang, M. G. Choi, Y. A. Jeong, S. H. Lee, and S. K. Chang, Tetrahedron Lett., 2017, 58, 474.
R. L. Sheng, P. F. Wang, Y. H. Gao, Y. Wu, W. M. Liu, J. J. Ma, H. P Li, and S. K. Wu, Org. Lett., 2008, 10, 5015.
X. Y. Xue, D. Y. Jiang, C. Feng, H. Zhang, Z. F. Wang, and H. Zhao, Inorg. Chem. Commun., 2017, 86, 258.
W. Li, Y. Zhang, X. P. Gan, M. D. Yang, B. Mie, M. Fang, Q. Y. Zhang, J. H. Yu, J. Y. Wu, Y. P. Tian, and H. P. Zhou, Sens. Actuators, B, 2015, 206, 640.
A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891.
X. Qi, E. J. Jun, L. Xu, S.-J. Kim, J. S. J. Hong, Y. J. Yoon, and J. Y. Yoon, J. Org. Chem, 2006, 71, 2881.
D. C. Wang, J. L. Fan, X. Q. Gao, B. S. Wang, S. G. Sun, and X. J. Peng, J. Org. Chem., 2009, 74, 7675.
Y. H. Yu, N. Bogliotti, J. Tang, and J. Xie, Eur. J. Org. Chem., 2013, 2013, 7749.
J. S. Kim and D. T. Quang, Chem. Rev., 2007, 107, 3780.
A. Fujii, Y. Sekiguchi, H. Matsumura, T. Inoue, W. S. Chung, S. Hirota, and T. Matsuo, Bioconjugate Chem., 2015, 26, 537.
M. Danko, J. Kollár, M. Cigán, Š. Chmela, and P Hrdlovic, Dyes Pigm., 2018, 153, 189.
A. J. Howarth, M. B. Majewski, and M. O. Wolf, Coord. Chem. Rev., 2015, 282-283, 139.
X. Cheng, H. Jia, T. Long, J. Feng, J. Qin, and Z. Li, Chem. Commun., 2011, 47, 11978.
M. Üçüncü and M. Emrullahoglu, Chem. Commun., 2014, 50, 5884.
Y. Wang, J. C. Xia, J. Han, X. Bao, Y. Y. Li, X. Tang, L. Ni, L. Wang, and M. M. Gao, Talanta, 2016, 16, 847.
Y. Yang, C. Y. Gao, J. Chen, N. Zhang, and D. W. Dong, Anal. Methods, 2016, 8, 805.
Y. H. Yu, T T Shu, B. J. Yu, Y. Deng, C. Fu, Y. G. Gao, C. Z. Dong, and Y. B. Ruan, Sens. Actuators, B, 2018, 255, 3170.
L. Y. Zong, C. Wang, Y. C. Song, Y. J. Xie, P. Zhang, Q. Peng, Q. Q. Li, and Z. Li, Sens. Actuators, B, 2017, 252, 1105.
R. V. Rathod, S. Bera, M. Singh, and D. Mondai, RSC Adv., 2016, 6, 34608.
J. W. Yoon, M. J. Chang, S. Hong, and M. H. Lee, Tetrahedron Lett., 2017, 58, 3887.
Y. C. Li, X. J. Han, and Y. Song, RSC Adv., 2017, 7, 20537.
H. S. Kumbhar, B. L. Gadilohar, and G. S. Shankarling, Sens. Actuators, B, 2016, 222, 35.
C. J. Wu, J. B. Wang, J. J. Shen, C. Y. Zhang, Z. Y. Wu, and H. W. Zhou, Tetrahedron, 2017, 73, 5715.
Y. W. Ma, T H. Leng, Y. R. Qu, C. Y. Wang, Y. J. Shen, and W. H. Zhu, Tetrahedron, 2017, 73, 14.
G. K. Patra, R. Chandra, A. Ghorai, and K. K. Shrivas, Inorg. Chim. Acta, 2017, 462, 315.
S. Y. Lee and C. Kim, Inorg. Chem. Commun., 2017, 77, 6.
X. Wang, W. Shi, L. Feng, J. C. Ma, Y. Q. Li, X. J. Kong, Y. B. Chen, Y. H. Hui, and Z. F. Xie, Inorg. Chem. Commun., 2017, 79, 50.
J. B. Wang and Q. S. Zong, Sens. Actuators, B, 2015, 216, 572.
J. B. Li, S. Chen, P. Zhang, Z. Wang, G. K. Long, R. Ganguly, Y. X. Li, and Q. C. Zhang, Chem. Asian J., 2016, 11, 136.
Y. Feng, S. Q. Li, D. X. Li, Q. Wang, P. Ning, M. Chen, X. H. Tian, and X. Wang, Sens. Actuators, B, 2018, 254, 282.
L. Yuan, W. Y. Lin, J. Z. Song, and Y. T. Yang, Chem. Commun., 2011, 47, 12691.
Y. Zhao, H. Y. Li, Y. Y. Xue, Y. H. Ren, and T. H. Han, Sens. Actuators, B, 2017, 241, 335.
G. Y. Li, Q. Lin, L. N. Ji, and H. Chao, J. Mater. Chem. B, 2014, 2, 7918.
G. Y. Li, Q. Lin, L. L. Sun, C. S. Feng, P. Y. Zhang, B. L. Yu, Y. Chen, Y. Wen, H. Wang, L. N. Ji, and H. Chao, Biomaterials, 2015, 53, 285.
S. Goswami, S. Paul, and A. Manna, Dalton Trans., 2013, 42, 10097.
S. Goswamia, S. Maitya, A. C. Maitya, and A. K. Das, Sens. Actuators, B, 2014, 204, 741.
D. X. Li, X. Sun, J. M. Huang, Q. Wang, Y. Feng, M. Chen, X. M. Meng, M. Z. Zhu, and X. Wang, Dyes Pigm., 2016, 125, 185.
B. Ritochvil, D. A. Zatko, and R. Markuszewski, Anal. Chem., 1966, 38, 770.
A. F. Li, H. He, Y. B. Ruan, Z. C. Wen, J. S. Zhao, Q. J. Jiang, and Y. B. Jiang, Org. Biomol. Chem., 2009, 7, 193.
K. Mariappan, M. Alaparthi, G. Caple, V. Balasubramanian, M. M. Hoffman, M. Hudspeth, and A. G. Sykes, Inorg. Chem., 2014, 53, 2953.
Acknowledgments
The authors thank National Natural Science Foundation of China (No. 21708015) for supporting this work.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Shu, T., Deng, X., Dong, C. et al. Diaminomaleonitrile-based Fluorophores as Highly Selective Sensing Platform for Cu2+. ANAL. SCI. 35, 987–993 (2019). https://doi.org/10.2116/analsci.19P117
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.19P117