Abstract
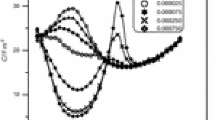
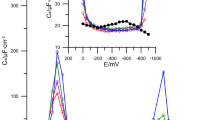
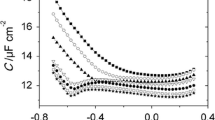
The adsorption–desorption phenomena of surfactants were studied by measuring differential capacity–potential curves in a static solution and differential capacity–time curves in a flowing solution. The surfactants investigated were Aerosol OT, cetylpyridinium chloride, Hyamin 1622, tetrabutylammonium bromide, Triton X-100 and trioctylphosphineoxide. The differential capacity–potential and differential capacity–time curves for these surfactants showed different shapes, with and without peaks. The differential capacity–time curves were used to study the adsorption reversibility of the surfactants at a mercury electrode. The adsorptions of Hyamin 1622 and Triton X-100 were irreversible at all the potentials investigated. The adsorptions of Aerosol OT and trioctylphosphineoxide were irreversible except at the potential more positive than–0.2 V. The adsorption of tetrabutylammonium bromide was almost reversible at any potential investigated. The adsorption of cetylpyridinium chlolide was complicated. indicating different orientations of adsorption.
Similar content being viewed by others
References
I. Kobayashi, Bunseki, 1995, 2, 123.
M. Gerlachet, J. M. Kauffmann, G. Quarin, J. C. Vire, G. A. Bryant, and J. M. Talbot, Talanta, 1996, 43, 507.
A. Szymanski and Z. Lukaszewski, Anal. Chim. Acta, 1992, 260, 25.
B. Wyrwas, A. Szymanski, and Z. Lukaszewski, Talanta, 1995, 42, 1251.
N. Batina and B. Cosovic, J. Electroanal. Chem., 1987, 227, 129.
J.-L. Besombes, G. Mousset, and C. Mousty, J. Electroanal. Chem., 1993, 349, 127.
P. Nikitas and S. Sotiropoulos, J. Electroanal. Chem., 1991, 309, 1.
M. Rueda, A. Moto, M. L. S. Goncalues, I. Navarro, and F. Svestka, J. Electroanal. Chem., 1997, 431, 257.
L. Pospisil and M. Svestka, J. Electroanal. Chem., 1997, 426, 47.
V. Stauffer, R. Stoodley, J. O. Agak, and D. Bizzotto, J. Electroanal. Chem., 2001, 516, 73.
B. B. Damaskin, O. A. Baturina, E. V. Stenina, and L. N. Sviridova, Electrochim. Acta, 2001, 46, 3091.
H. Sawamoto and K. Gamoh, J. Electroanal. Chem., 1990, 28, 421.
H. Sawamoto, J. Electroanal. Chem., 1993, 361, 215.
H. Sawamoto, J. Electroanal. Chem., 1994, 375, 391.
H. Sawamoto, Nippon Kagaku Kaishi, 1996, 958.
H. Sawamoto, Nippon Kagaku Kaishi, 1997, 294.
H. Sawamoto, J. Electroanal. Chem., 1997, 432, 153.
H. Sawamoto, Anal. Sci., 1999, 15, 73.
H. Sawamoto, Nippon Kagaku Kaishi, 1998, 511.
S. Trasatti, Electrochim. Acta, 1992, 37, 2137.
G. M. Torrie and G. N. Patey, Electrochim. Acta, 1991, 36, 1677.
Z. Q. Tian, S. K. Sigalaev, S. Z. Zou, B. W. Mao, A. M. Funtikov, and V. E. Kazarinov, Electrochim. Acta, 1994, 39, 2195.
J. G. Gordon, O. R. Melroy, and M. F. Toney, Electrochim. Acta, 1995, 40, 3.
T. Matsui, Denki Kagaku, 1995, 63, 275.
E. Spohr, G. Toth, and K. Heinzinger, Electrochim. Acta, 1996, 41, 2131.
M. C. Dubey and M. Singh, Indian J. Chem., Sect. A, 1979, 18A, 2, 174; CA 92:66751b.
A. Varshney, M. Krishna, S. K. Jha, and M. Singh, J. Indian Chem. Soc., 1980, 57, 816; CA 93:17991w.
S. L. Gupta and S. K. Sharma, Electrochim. Acta, 1965, 10, 151.
S. K. Sharma, Electrochim. Acta, 1971, 16, 517.
M. K. Kaisheva and V. K. Kaishev, God. Sofii. Univ., Khim. Fak, 1979, 70, Pt.2, 71; CA 93:56860t.
N. Gundersen and E. Jacobsen, J. Electroanal. Chem., 1969, 20, 13.
M. Hamdi, R. Bennes, D. Schuhmann, and P. Vanel, J. Electroanal. Chem., 1980, 108, 255.
M. A. V. Devanathan and M. J. Fernando, Trans. Faraday Soc., 1962, 58, 368.
L. Nemec, Collection Czechoslov. Chem. Commun., 1966, 31, 1162.
T. Ohsaka, H. Kato, and T. Yoshida, Denki Kagaku, 1973, 41, 156.
T. Ohsaka, H. Yamamoto, and T. Yoshida, Bull. Chem. Soc. Jpn., 1973, 46, 1320.
H.-D. Dorfler and E. Muller, J. Electroanal. Chem., 1979, 105, 383.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sawamoto, H. Reversible and Irreversible Adsorption of Surfactants at a Hanging Mercury Drop Electrode. ANAL. SCI. 19, 1381–1386 (2003). https://doi.org/10.2116/analsci.19.1381
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.19.1381