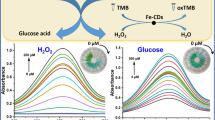
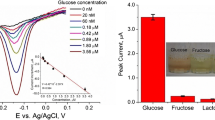
Abstract
In this work, umbelliferone, a kind of coumarin derivative, was proved to exhibit peroxidase-like activity that could catalyze the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of hydrogen peroxide to generate a blue-colored oxide (oxTMB). The catalytic mechanism is similar to that of native enzymes (e.g. horseradish peroxidase, HRP) and nanozymes, which follow the Michaelis–Menten kinetics behavior. Meanwhile, the 7-hydroxyl group of umbelliferone plays a significant role in the peroxidase-like activity. Compared with enzymes and nanozymes, this small molecular mimic enzyme possesses the advantages of low cost, simple molecular structures, small molecular weight and high stability against harsh conditions. Based on the favorable peroxidase mimetic activity of umbelliferone, a convenient, practical and sensitive H2O2 and glucose detection method was successfully established. This work not only opens some new inspirations into seeking for novel molecular enzyme mimetics with excellent catalytic activities, but also provides promising assays for clinical diagnosis.
Similar content being viewed by others
References
I. X. Green, W. Tang, M. McEntee, M. Neurock, and J. T. Yates, Jr., J. Am. Chem. Soc., 2012, 134, 12717.
H. G. Yang, J. Q. Zha, P. Zhang, Y. H. Xiong, L. J. Su, and F. G. Ye, RSC Adv., 2016, 6, 66963.
J. Z. Chen, Y. J. Liu, G. X. Zhu, and A. H. Yuan, Cryst. Res. Tenchol., 2014, 49, 309.
R. Akter, C. K. Rhee, and M. A. Rahman, Biosens. Bioelectron., 2015, 66, 539.
L. J. Su, Y. H. Xiong, H. H. Yang, P. Zhang, and F. G. Ye, J. Mater. Chem. B, 2016, 4, 128.
L. Z. Gao, J. Zhuang, L. Nie, J. B. Zhang, Y. Zhang, N. Gu, T. H. Wang, J. Feng, D. L. Yang, S. Perrett, and X. Y. Yan, Nat. Nanotechnol., 2007, 2, 577.
E. Nakamura and H. Isobe, Acc. Chem. Rev., 2003, 36, 807.
Y. N. Ding, B. C. Yang, H. Liu, Z. X. Liu, X. Zhang, X. W. Zheng, and Q. Y. Liu, Sens. Actuators, B, 2018, 259, 775.
Y. F. Wang, N. Pan, and C. F. Peng, Anal. Sci., 2017, 33, 321.
Q. Y. Liu, Y. T. Yang, H. Li, R. R. Zhu, Q. Shao, S. G. Yang, and J. J. Xu, Biosens. Bioelectron., 2015, 64, 147.
Q. Y. Liu, Y. T. Yang, X. T. Lv, Y. N. Ding, Y. Z. Zhang, J. J. Jing, and Ch. X. Xu, Sens. Actuators, B, 2017, 240, 726.
Z. Z. Yang, F. Q. Ma, Y. Zhu, S. H. Chen, C. Wang, and X. F. Lu, Dalton Trans., 2017, 46, 11171.
F. Kang, X. S. Hou, and K. Xu, Nanotechnology, 2015, 26, 405707.
E. Kuah, S. Toh, J. Yee, Q. Ma, and Z. Q. Gao, Chemistry, 2016, 22, 8404.
L. Liu, Y. Shi, Y. F. Yang, M. L. Li, Y. J. Long, Y. M. Huang, and H. Z. Zheng, Chem. Commun., 2016, 52, 13912.
R. Simkovitch and D. Huppert, J. Phys. Chem. B, 2015, 119, 14683.
F. Wu and S. Sheu, Chin. Pharm. J., 1992, 44, 257.
C. M. Krauter, J. Mohring, T. Buckup, M. Pernpointner, and M. Motzkus, Phys. Chem. Chem. Phys., 2013, 15, 17846.
C. C. Perry, V. J. Tang, K. M. Konigsfeld, J. A. Aguilera, and J. R. Milligan, J. Phys. Chem. B, 2011, 115, 9889.
W. Y. Zhai, C. X. Wang, P. Yu, Y. X. Wang, and L. Q. Mao, Anal. Chem., 2014, 86, 12206.
L. Pan, X. Z. Li, Z. Q. Yan, H. R. Guo, and B. Qin, Plant Physiol. Biochem., 2015, 97, 272.
B. Garg and T. Bisht, Molecules, 2016, 21.
S. Kandil, A. D. Westwell, and C. McGuigan, Bioorg. Med. Chem. Lett., 2016, 26, 2000.
P. D. Josephy, T. Eling, and R. P. Mason, J. Biol. Chem., 1982, 257, 3669.
Z. Z. Yang, Y. Zhu, G. D. Nie, M. X. Li, C. Wang, and X. F. Lu, Dalton Trans., 2017, 46, 8942.
H. H. Zhi, J. D. Wang, S. J. Wang, and Y. J. Wei, J. Spectrosc., 2013, Article ID 147128.
J. Zhang, C. G. Liu, and Y. J. Wei, Huaxue Tongbao, 2011, 74, 957.
P. Ju, Y. Z. Yu, M. Wang, Y. Zhao, D. Zhang, C. J. Sun, and X. X. Han, J. Mater. Chem. B, 2016, 4, 6316.
Y. F. Yang, D. J. Shen, Y. J. Long, Z. X. Xie, and H. Z. Zheng, Sci. Rep., 2017, 7, 43141.
L. Su, W. Qin, H. Zhang, Z. U. Rahman, C. Ren, S. Ma, and X. Chen, Biosens. Bioelectron., 2015, 63, 384.
H. Wei and E. Wang, Anal. Chem., 2008, 80, 2250.
F. M. Qiao, L. J. Chen, X. N. Li, L. F. Li, and S. Y. Ai, Sens. Actuators, B, 2014, 193, 255.
Q. Chen, J. Chen, C. J. Gao, M. L. Zhang, J. Y. Chen, and H. D. Qiu, Analyst, 2015, 140, 2857.
C. M. Riccardi, D. Mistri, O. Hart, M. Anuganti, Y. Lin, R. M. Kasi, and C. V. Kumar, Chem. Commun., 2016, 52, 2593.
Z. Q. Yan, D. D. Wang, H. Y. Cui, D. H. Zhang, Y. H. Sun, H. Jin, X. Z. Li, X. Y. Yang, H. R. Guo, X. F. He, L. Pan, X. Ren, K. Guo, and B. Qi, Acta Physiol. Plant, 2016, 38, 248.
L. Su, X. A. Yu, W. J. Qin, W. P. Dong, C. K. Wu, Y. Zhang, G. J. Mao, and S. L. Feng, J. Mater. Chem. B, 2017, 5, 116.
S. H. Lim, J. Wei, J. Lin, Q. Li, and K. Jin, Biosens. Bioelectron., 2005, 11, 2341.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21405124, 21175110), the Fundamental Research Funds for the Central Universities (No. XDJK2013A022).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sun, C., Huang, Z., Liu, L. et al. Umbelliferone as a Small Molecular Peroxidase Mimic towards Sensitive Detection of H2O2 and Glucose. ANAL. SCI. 34, 933–938 (2018). https://doi.org/10.2116/analsci.18P023
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.18P023