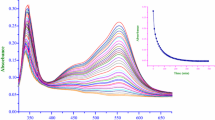
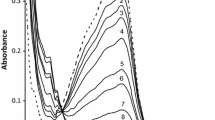
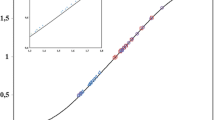
Abstract
The rate of formation/extraction of iron(III) with trifluoroacetylacetone in Triton X-100 micellar solution at 298 K was measured by stopped-flow spectrophotometry to identify the route and mechanism of the extraction process. The rate was first order with respect to the metal ion and the extractant in the bulk aqueous phase and inverse first order to the hydrogen ion when the formation of extractable tris-complex was small. The rate obtained under such conditions agreed well with that of complex formation in the single aqueous solution; this suggests that the whole reactions is controlled only by the formation of the mono-complex in the bulk aqueous phase. On the other hand, the rate did not show clear dependencies on the ligand and the hydrogen ion, and was slower than the one expected from the complex formation in the aqueous solution when the formation of the extractable tris-complex was dominant. A slow material transport of the tris-complex from the bulk aqueous phase to the micellar pseudophase was estimated.
Similar content being viewed by others
References
J. H. Fendler and E. J. Fendler, “Catalysis in Micellar and Macromolecular Systems”, 1975, Chap. 2, Academic Press, New York.
H. Watanabe, “Solution Chemistry of Surfactants”, ed. K. L. Mittal and E. J. Fendler, 1982, Vol. II, Plenum Press, New York, 1305.
J. Georges, Spectrochim. Acta Rev., 1990, 13, 27.
J. Szymanowski and C. Tondre, Solvent Extr. Ion Exch., 1994, 12, 873.
J. G. Huddleston, H. D. Willauer, S. T. Griffin, and R. D. Rogers, Ind. Eng. Chem. Res., 1999, 38, 2523.
K. Hayashi, Y. Sasaki, S. Tagashira, and E. Kosaka, Anal. Chem., 1986, 58, 1444.
R. P. Paradkar and R. R. Williams, Anal. Chem., 1994, 66, 2752.
B. R. Fillipi, J. F. Scamehorn, S. D. Christian, and R. W. Taylor, J. Membrane Sci., 1998, 145, 27.
H. Watanabe, T. Saitoh, T. Kamidate, and K. Haraguchi, Mikrochim. Acta, 1992, 106, 83.
S. Muralidharan, W. Yu, S. Tagashira, and H. Freiser, Langmuir, 1990, 6, 1190.
S.-G. Son, M. Hebrant, P. Tecilla, P. Scrimin, and C. Tondre, J. Chem., 1992, 96, 1 1072.
K. Inaba, S. Muralidharan, and H. Freiser, Anal. Chem., 1993, 65, 1510.
J. K. McCulloch, D. Fornasiero, J. M. Perera, B. S. Murray, G. W. Stevens, and F. Grieser, J. Colloid Interface Sci., 1993, 157, 180.
T. Saitoh, H. Hoshino, and T. Yotsuyanagi, J. Chem. Soc. Faraday Trans., 1994, 90, 479.
J. Szymanowski, “Value Adding Through Solvent Extraction”, ed. D. C. Shallcross, R. Paimin, and L. M. Prvcic, 1996, Vol. I, University of Melbourne, Melbourne, 219.
G. Ma, H. Freiser, and S. Muralidharan, Anal. Chem., 1997, 69, 2827.
K. Inaba, “Value Adding Through Solvent Extraction”, ed. D. C. Shallcross, R. Paimin, and L. M. Prvcic, 1996, Vol. I, University of Melbourne, Melbourne, 57.
K. Inaba, Langmuir, 1997, 13, 1501.
K. Inaba, unpublished data.
K. Kalyanasundaram and J. K. Thomas, J. Am. Chem. Soc., 1977, 99, 2039.
I. V. Berezin, K. Martinek, and A. K. Yatsimirskii, Russ. Chem. Rev., 1973, 42, 787.
L. Ciavatta and M. Grimaldi, J. Inorg. Nucl. Chem., 1975, 37, 163.
T. Sekine and K. Inaba, Bull. Chem. Soc. Jpn., 1982, 55, 3773.
K. Inaba, N. Itoh, Y. Matsuno, and T. Sekine, Bull. Chem. Soc. Jpn., 1985, 58, 2176.
K. Inaba and T. Sekine, Anal. Sci., 1987, 3, 117.
K. Inaba, Anal. Sci., Part II, in this issue.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inaba, K. Unusually Slow Extraction Rate and Mechanism of Iron(III) with Trifluoroacetylacetone in Triton X-100 Micellar System. Part I. Extraction Routes. ANAL. SCI. 16, 811–817 (2000). https://doi.org/10.2116/analsci.16.811
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.16.811