Abstract
Objective
In the province of Manitoba, Canada, given that latent tuberculosis infection (LTBI) treatment is provided at no cost to the patient, treatment completion rates should be optimal. The objective of this study was to estimate LTBI treatment completion using prescription drug administrative data and identify patient characteristics associated with completion.
Methods
Prescription drug data (1999–2014) were used to identify individuals dispensed isoniazid (INH) or rifampin (RIF) monotherapy. Treatment completion was defined as being dispensed INH for ≥ 180 days (INH180) or ≥ 270 days (INH270) or RIF for ≥ 120 days (RIF120). Logistic regression models tested socio-demographic and comorbidity characteristics associated with treatment completion.
Results
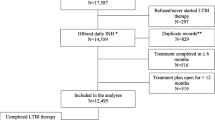
The study cohort comprised 4985 (90.4%) persons dispensed INH and 529 (9.6%) RIF. Overall treatment completion was 60.2% and improved from 43.1% in 1999–2003 to 67.3% in 2009–2014. INH180 showed the highest completion (63.8%) versus INH270 (40.4%) and RIF120 (27.0%). INH180 completion was higher among those aged 0–18 years (68.5%) compared with those aged 19+ (61.0%). Sex, geography, First Nations status, income quintile, and comorbidities were not associated with completion.
Conclusions
Benchmark 80% treatment completion rates were not achieved in Manitoba. Factors associated with non-completion were older age, INH270, and RIF120. Access to shorter LTBI treatments, such as rifapentine/INH, may improve treatment completion.
Résumé
Objectifs
Au Manitoba, Canada, étant donné que le traitement de l’infection tuberculeuse latente (ITL) est gratuit, le taux d’achèvement du traitement devrait être optimal. L’objectif de cette étude était d’estimer l’achèvement du traitement de l’ITL à l’aide de données administratives sur les médicaments d’ordonnance et d’identifier les caractéristiques des patients associées à l’achèvement du traitement.
Méthodes
Les données sur les médicaments d’ordonnance (1999 à 2014) ont été utilisées pour identifier les personnes dispensées en monothérapie l’isoniazide (INH) ou la rifampicine (RIF). L’achèvement du traitement a été définie comme étant une délivrance d’INH supérieure ou égale à 180 jours (INH180) ou supérieure ou égale à 270 jours (INH270) ou une délivrance de la RIF supérieure ou égale à 120 jours (RIF120). Les modèles de régression logistique ont évalué les caractéristiques sociodémographiques et de comorbidité associées à l’achèvement du traitement.
Résultats
La cohorte à l’étude comprenait 4985 (90,4 %) individus dispensées d’INH et 529 (9,6 %) de la RIF. L’achèvement du traitement globale était de 60,2 % et est passé de 43,1 % en 1999–2003 jusqu’à 67,3 % en 2009–2014. L’INH180 a montré l’achèvement le plus élevé (63,8 %) par rapport à l’INH270 (40,4 %) et à la RIF120 (27,0 %). L’achèvement de l’INH180 était plus élevé chez les 0 à 18 ans (68,5 %) que chez les 19 ans et plus (61,0 %). Le sexe, la région de résidence, l’identification des Premières nations, le quintile de revenu et les comorbidités n’étaient pas associés à l’achèvement du traitement.
Conclusions
Le taux de référence du traitement de 80 % n’a pas été atteint au Manitoba. Les facteurs associés au non-achèvement étaient l’âge et l’utilisation d’INH270 ou de la RIF120. L’accès à des traitements plus courts de l’ITL, tels que la rifapentine/INH, pourrait améliorer l’achèvement du traitement.



Similar content being viewed by others
References
Alsdurf, H., Hill, P. C., Matteelli, A., et al. (2016). The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. The Lancet Infectious Diseases, 16(11), 1269–1278.
Alvarez, G. G., VanDyk, D. D., Aaron, S. D., et al. (2014). TAIMA (stop) TB: the impact of a multifaceted TB awareness and door-to-door campaign in residential areas of high risk for TB in Iqualuit, Nunavut. PLoS One, 9(7), e100975. https://doi.org/10.1371/journal.pone.0100975.
Aspler, A., Long, R., Trajman, A., et al. (2010). Impact of treatment completion, intolerance and adverse events on health system costs in a randomised trial of 4 months rifampin or 9 months isoniazid for latent TB. Thorax., 65, 582–587.
Charlson, M. E., Pompei, P., Ales, K. L., & MacKenzie, C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases, 40(5), 373–383.
Gallant, V., Duvvuri, V., & McGuire, M. (2017). Tuberculosis in Canada – summary 2015. Canada Communicable Disease Report, 43(3), 77–82.
Health Canada, Drugs and Health Products, Access to Drugs in Exceptional Circumstances. List of drugs for an urgent public health need. February 2018. (accessed May 2019 at https://www.canada.ca/en/health-canada/services/drugs-health-products/access-drugs-exceptional-circumstances/list-drugs-urgent-public-health-need.html).
Hirsch-Moverman, Y., Shrestha-Kuwahara, R., Bethel, J., et al. (2015). Latent tuberculous infection in the United States and Canada: who completes treatment and why? The International Journal of Tuberculosis and Lung Disease, 19(1), 31–38.
Horsburgh, C. R., Jr., Goldberg, S., Bethel, J., et al. (2010). Latent TB infection treatment acceptance and completion in the United States and Canada. Chest, 137(2), 401–409.
Malejczyk, K., Gratrix, J., Beckon, A., et al. (2014). Factors associated with noncompletion of latent tuberculosis infection treatment in an inner-city population in Edmonton, Alberta. The Canadian Journal of Infectious Diseases & Medical Microbiology AMMI Canada, 25(5), 281–284.
Manitoba Health, Seniors and Active Living. Manitoba Tuberculosis Protocol. February 2014. (accessed May 2019 at http://www.gov.mb.ca/health/publichealth/cdc/protocol/tb.pdf).
Pettit, A. C., Bethel, J., Hirsch-Moverman, Y., Colson, P. W., & Sterling, T. R. (2013). Female sex and discontinuation of isoniazid due to adverse effects during the treatment of latent tuberculosis. Journal of Information Security, 67(5), 424–432.
Public Health Agency of Canada and the Canadian Thoracic Society and the Canadian Lung Association. Canadian Tuberculosis Standards. 7th edition. February 2014. (accessed May 2019 at http://www.phac-aspc.gc.ca/tbpc-latb/pubs/tb-canada-7/index-eng.php).
Rivest, P., Street, M. C., & Allard, R. (2013). Completion rates of treatment for latent tuberculosis infection in Quebec, Canada from 2006 to 2010. Canadian Journal of Public Health, 104(3), e235–e239.
Rubinowicz, A., Bartlett, G., MacGibbon, B., et al. (2014). Evaluating the role of primary care physicians in the treatment of latent tuberculosis: a population study. The International Journal of Tuberculosis and Lung Disease, 18(12), 1449–1454.
Smith, B. M., Schwartzman, K., Bartlett, G., & Menzies, D. (2011). Adverse events associated with treatment of latent tuberculosis in the general population. CMAJ, 183(3), E173–E179.
Spicer, K. B., Perkins, L., DeJesus, B., et al. (2013). Completion of latent tuberculosis therapy in children: impact of country of origin and neighborhood clinics. Journal of the Pediatric Infectious Diseases Society, 2(4), 312–319.
Stagg, H. R., Zenner, D., Harris, R. J., et al. (2014). Treatment of latent tuberculosis infection: a network meta-analysis. Annals of Internal Medicine, 161, 419–428. https://doi.org/10.7326/M14-1019.
Sterling, T. R., Villarino, M. E., Borisov, A. S., et al. (2011). TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. The New England Journal of Medicine, 365, 2155–2166.
Acknowledgements
We acknowledge the Manitoba Centre for Health Policy (MCHP) for the use of the data in the Manitoba Population Research Data Repository under HIPC project #2015/2016-64.
Financial support
This study was financially funded by the operational funds from the Province of Manitoba and the Manitoba Centre for Health Policy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval was provided by the University of Manitoba Health Research Ethics Board and the Manitoba First Nations Health Information Governance Committee, which oversees health research involving First Nations. Approval for data access was provided by the Manitoba Health Information Privacy Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy or Manitoba Health, Seniors and Active Living is intended or should be inferred.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Plourde, P.J., Basham, C.A., Derksen, S. et al. Latent tuberculosis treatment completion rates from prescription drug administrative data. Can J Public Health 110, 705–713 (2019). https://doi.org/10.17269/s41997-019-00240-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.17269/s41997-019-00240-1