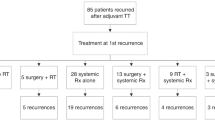
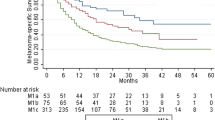
Abstract
Background
The survival of patients with melanoma of unknown primary (MUP) is proposed to be more favourable than that for melanoma of known primary (MKP). This may be due to an enhanced initial immune response in patients with MUP, which could also affect the efficacy of immunotherapy in advanced disease.
Objectives
The present study compared therapeutic outcome and survival in Stage III and IV MUP and MKP.
Results
Medical records of 67 MUP and 536 MKP patients were reviewed. Median overall survival (OS) in Stage III patients was 77 months versus 54 months in patients with MUP and MKP, respectively (p = 0.11). Median OS was prolonged in MUP patients receiving adjuvant first-line ipilimumab (p = 0.14). In contrast, OS tended to be more favourable in patients with MKP after palliative first-line ipilimumab treatment (p = 0.16). Yet, no statistically significant differences in OS were detected between the groups. Moreover, survival after anti-PD-1-antibody treatment was similar in patients with MUP and MKP.
Conclusion
Overall, we observed similar survival outcomes after immunotherapy in patients with MUP and MKP. These findings provide no evidence of difference in responsiveness to immunotherapy between patients with MUP and MKP.
Similar content being viewed by others

References
Kamposioras K, Pentheroudakis G, Pectasides D, Pavlidis N. Malignant melanoma of unknown primary site. To make the long story short. A systematic review of the literature. Crit Rev Oncol Hematol 2011; 78:112–26.
Bae JM, Choi YY, Kim DS, et al. Metastatic melanomas of unknown primary show better prognosis than those of known primary: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol 2015;72:59–70.
Das Gupta TBL, Berg JW. Malignant melanoma of unknown primary origin. Surg Gynecol Obstet 1963; 117: 341–5.
Egberts F, Bergner I, Kruger S, et al. Metastatic melanoma of unknown primary resembles the genotype of cutaneous melanomas. Ann Oncol 2014;25:246–50.
Dutton-Regester K, Kakavand H, Aoude LG, et al. Melanomas of unknown primary have a mutation profile consistent with cutaneous sun-exposed melanoma. Pigment cell Melanoma Res 2013; 26: 852–60.
Anbari KK, Schuchter LM, Bucky LP, et al. Melanoma of unknown primary site: presentation, treatment, and prognosis-a single institution study. University of Pennsylvania Pigmented Lesion Study Group. Cancer 1997; 79: 1816–21.
de Waal AC, Aben KK, van Rossum MM, Kiemeney LA. Melanoma of unknown primary origin: a population-based study in the Netherlands. Eur J Cancer 2013;49:676–83.
Scott JF, Conic RZ, Thompson CL, Gerstenblith MR, Bordeaux JS. Stage IV melanoma of unknown primary: a population-based study in the United States from 1973 to 2014. J Am Acad Dermatol 2018;79:258–65.e4.
Schlagenhauff B, Stroebel W, Ellwanger U, et al. Metastatic melanoma of unknown primary origin shows prognostic similarities to regional metastatic melanoma: recommendations for initial staging examinations. Cancer 1997; 80: 60–5.
Kuk D, Shoushtari AN, Barker CA, et al. Prognosis of mucosal, uveal, acral, nonacral cutaneous, and unknown primary melanoma from the time of first metastasis. Oncologist 2016; 21: 848–54.
Smith JL Jr., Stehlin JS Jr.. Spontaneous regression of primary malignant melanomas with regional metastases. Cancer 1965;18:1399–415.
Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology 1992; 20: 315–22.
Cervinkova M, Kucerova P, Cizkova J. Spontaneous regression of malignant melanoma — is it based on the interplay between host immune system and melanoma antigens?. Anticancer Drugs 2017; 28: 819–30.
Aung PP, Nagarajan P, Prieto VG. Regression in primary cutaneous melanoma: etiopathogenesis and clinical significance. Lab Invest 2017. doi: https://doi.org/10.1038/labinvest.2017.8.
Heppt MV, Tietze JK, Reinholz M, et al. Disease kinetics butnotdis-ease burden is relevant for survival in melanoma of unknown primary tumor. Discov Med 2015;20:231–7.
Ridolfi RL, Rosen PP, Thaler H. Nevus cell aggregates associated with lymph nodes: estimated frequency and clinical significance. Cancer 1977; 39: 164–71.
Shenoy BV, Fort L, 3rd L, Benjamin SP. Malignant melanoma primary in lymph node. The case of the missing link. Am J Surg Pathol 1987; 11:1406.
Carson KF, Wen DR, Li PX, et al. Nodal nevi and cutaneous melanomas. Am J Surg Pathol 1996; 20: 83440.
van der Ploeg AP, Haydu LE, Spillane AJ, et al. Melanoma patients with an unknown primary tumor site have a better outcome than those with a known primary following therapeutic lymph node dissection for macroscopic (clinically palpable) nodal disease. Ann Surg Oncol 2014; 21: 3108–16.
McCarthy SW, Palmer AA, Bale PM, Hirst E. Naevus cells in lymph nodes. Pathology 1974; 6: 351–8.
Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, Phase 2 trial. Lancet Oncol 2015; 16:908–18.
Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label Phase 2 study. Lancet Oncol 2013; 14: 249–56.
Dummer R, Schadendorf D, Ascierto PA, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, Phase 3 trial. Lancet Oncol 2017;18:435–45.
Dummer R, Schadendorf D, Ascierto PA, Larkin J, Lebbe C, Hauschild A. Integrating first-line treatment options into clinical practice: what’s new in advanced melanoma?. Melanoma Res 2015; 25: 461–9.
Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in Stage III melanoma with ipilimumab adjuvant therapy. New Eng J Med 2016; 375:1845–55.
Arozarena I, Sanchez-Laorden B, Packer L, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer cell 2011; 19: 45–57.
Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. New Engl J Med 2015; 372: 2521–32.
Mangana J, Cheng PF, Kaufmann C, et al. Multicenter, real-life experience with checkpoint inhibitors and targeted therapy agents in advanced melanoma patients in Switzerland. Melanoma Res 2017; 27:358–68.
Kaunitz GJ, Cottrell TR, Lilo M, et al. Melanoma subtypes demonstrate distinct PD-L1 expression profiles. Lab Invest 2017; 97: 1063–71.
Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016;122:3344–53.
Bai X, Mao LL, Chi ZH, et al. BRAF inhibitors: efficacious and tolerable in BRAF-mutant acral and mucosal melanoma. Neoplasma 2017;64:626–32.
Alexander M, Mellor JD, McArthur G, Kee D. Ipilimumab in pretreated patients with unresectable or metastatic cutaneous, uveal and mucosal melanoma. Med J Australia 2014; 201: 49–53.
Utter K, Goldman C, Weiss SA, et al. Treatment outcomes for metastatic melanoma of unknown primary in the new era: a single-institution study and review of the literature. Oncology 2017; 93: 249–58.
Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCc Melanoma Staging and Classification. J Clin Oncol 2009; 27:6199–206.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45: 22847.
Varey E, Dalle S, Nguyen J, et al. Epidemiological study of unknown primary melanoma patients from the French national melanoma database RIC-Mel. J Clin Oncol 2018; e21571.
Network CGA. Genomic classification of cutaneous melanoma. Cell 2015; 161: 1681–96.
Krauthammer M, Kong Y, Ha BH, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet 2012; 44:1006–14.
Krauthammer M, Kong Y, Bacchiocchi A, et al. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat Genet 2015;47:996–1002.
Verver D, van der Veldt A, van Akkooi A, Verhoef C, Grünhagen DJ, Louwman WJ. Treatment of melanoma of unknown primary in the era of immunotherapy and targeted therapy: a Dutch population-based study. IntJ Cancer 2020; 146: 26–34.
Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer 2017; 86:37–45.
Robert C, Ribas A, Hamid O, Daud A, Wolchock JD, Joshua AM. Three-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol 2016; 34:9503.
Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label Phase III trial. J Clin Oncol 2018; 36: 383–90.
Omid H, Caroline R, Adil D, et al. Five-year survival outcomes in patients (pts) with advanced melanoma treated with pembrolizumab (pembro) in KEYNOTE-001. Ann Oncol 2019;30:582–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements and disclosures
Acknowledgements: we thank our colleagues from the Comprehensive Cancer Center Zürich (CCCZ) for providing insight in the Giessener tumour documenting system (GTDS) and assisting in data collection. Conflicts of interest: Joanna Mangana has intermittent project-focused consultant or advisory relationships with Merck/Pfizer and Pierre Fabre and receives travel support from Merck Sharp & Dohme und Pierre Fabre outside of the submitted work. Reinhard Dummer has intermittent, project-focused consulting and/or advisory relationships with Novartis, Merck Sharp & Dhome (MSD), Bristol-Myers Squibb (BMS), Roche, Amgen, Takeda, Pierre Fabre, Sun Pharma, and Sanofi outside the submitted work. Peter Koelblinger has received a research fellowship grant from the European Academy of Dermatology and Venerology and honoraria for travel support and consulting/advisory roles from Roche, Bristol-Myers Squibb (BMS), Merck Sharp & Dome (MSD), Novartis and Amgen outside the submitted work. Sabine Ludwig, Ralph Braun and Phil Cheng have declared no conflicts of interest.
Supplementary material
About this article
Cite this article
Ludwig, S.V., Cheng, P.F., Mangana, J. et al. Survival and therapeutic response in patients with melanoma of unknown and known primary: a single-centre retrospective analysis. Eur J Dermatol 30, 699–709 (2020). https://doi.org/10.1684/ejd.2020.3929
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1684/ejd.2020.3929