Abstract
Background
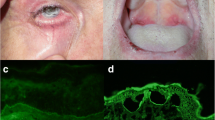
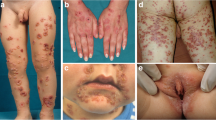
Mucosal involvement in autoimmune subepidermal blistering disorders (ASBD) may represent the only or predominant localization. Circulating autoantibodies are detected in 50% cases.
Objective
The aim of this study was to evaluate the usefulness of fluorescence overlay antigen mapping by laser-scanning confocal microscopy (FOAM-LSCM) to identify ASBD with mucosal involvement in oral mucosa specimens.
Materials & Methods
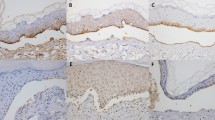
Thirty-two ASBD patients, diagnosed based on direct immunofluorescence between 2006 and 2016, were enrolled. Localization of IgG deposits bound at the basement membrane zone, relative to laminin-332 and collagen IV localization, was assessed in vivo.
Results
FOAM-LSCM disclosed four different immunofluorescence patterns. IgG deposits were located above laminin-332, as in bullous pemphigoid (BP-type), in 19% cases and co-localized with laminin-332 (anti-laminin-332-type) in 6% cases. IgG deposits were found below laminin-332 and above collagen IV (mucous membrane pemphigoid-type) in 59% cases, and below collagen IV (epidermolysis bullosa acquisita-type) in 16%. Circulating antibodies were found in 56% cases.
Conclusion
The FOAM-LSCM method should be used in order to obtain a definitive diagnosis of ASBD with mucosal involvement, particularly in the presence of negative circulating antibodies
Similar content being viewed by others
References
Amber KT, Murrell DF, Schmidt E, Joly P, Borradori L. Autoimmune subepidermal bullous diseases of the skin and mucosae: clinical features, diagnosis, and management. Clin Rev Allergy Immunol 2018; 54: 26–51.
Chan LS, Ahmed AR, Anhalt GJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol 2002; 138: 370–9.
Schmidt E, Zillikens D. Pemphigoid diseases. Lancet 2013; 381: 320–32.
Zillikens D. BP180 as the common autoantigen in blistering diseases with different clinical phenotypes. Keio J Med 2002; 51: 21–8.
Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol 2017; 13: 157–69.
Xu HH, Werth VP, Parisi E, Sollecito TP. Mucous membrane pemphigoid. Dent Clin North Am 2013; 57: 611–30.
Schmidt E, Skrobek C, Kromminga A, et al. Cicatricial pemphigoid: IgA and IgG autoantibodies target epitopes on both intra- and extracellular domains of bullous pemphigoid antigen 180. Br J Dermatol 2001; 145: 778–83.
Amber KT, Bloom R, Hertl M. A systematic review with pooled analysis of clinical presentation and immunodiagnostic testing in mucous membrane pemphigoid: association of anti-laminin-332 IgG with oropharyngeal involvement and the usefulness of ELISA. J Eur Acad Dermatol Venereol 2016; 30: 72–7.
Egan CA, Lazarova Z, Darling TN, Yee C, Cote T, Yancey KB. Anti-epiligrin cicatricial pemphigoid and relative risk for cancer. Lancet 2001; 357: 1850–1.
Vodegel RM, Jonkman MF, Pas HH, et al. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol 2004; 151: 112–8.
Wozniak K, Kazama T, Kowalewski C. A practical technique for differentiation of subepidermal bullous diseases: localization of in vivo-bound IgG by laser scanning confocal microscopy. Arch Dermatol 2003; 139: 1007–11.
Bean SF, Waisman M, Michal B, et al. Cicatricial pemphigoid: immunofluorescent studies. Arch Dermatol 1972; 106: 195–9.
Gammon WR, Kowalewski C, Chorzelski T, Kumar V, Briggaman RA, Beutner EH. Direct immunofluorescence studies of sodium chloride-separated skin in the differential diagnosis of bullous pemphigoid and epidermolysis bullosa acquisita. J Am Acad Dermatol 1990; 22: 664–70.
Hashimoto T, Tsuruta D, Koga H, et al. Summary of results of serological tests and diagnoses for 4774 cases of various autoimmune bullous diseases consulted to Kurume University. Br J Dermatol 2016; 175: 953–65.
Wozniak K, Waszczykowska E, Hashimoto T, et al. Anti-epiligrin cicatricial pemphigoid initially limited to the upper respiratory tract. Br J Dermatol 2006; 154: 779–81.
Jakubowska B, Kowalewski C, Ishii N, et al. Mucous membrane pemphigoid with severe stricture of the esophagus mediated by IgG and IgA autoantibodies to LAD-1. Eur J Dermatol 2015; 25: 510–2.
Wozniak K, Hashimoto T, Ishii N, et al. Fluorescence overlay antigen mapping using laser scanning confocal microscopy differentiates linear IgA bullous dermatosis from epidermolysis bullosa acquisita mediated by IgA. Br J Dermatol 2013; 168: 634–8.
Hofmann S, Thoma-Uszynski S, Hunziker T, et al. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomain. J Invest Dermatol 2002; 119: 1065–73.
Feliciani C, Joly P, Jonkman MF, et al. Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol 2015; 172: 867–77.
Cozzani E, Di Zenzo G, Calabresi V, et al. Autoantibody profile of a cohort of 78 Italian patients with pemphigoid mucous membrane: correlation between reactivity profile and clinical involvement. Acta Derm Venereol 2016; 96: 768–73.
Hayakawa T, Furumura M, Fukano H, et al. Diagnosis of oral mucous membrane pemphigoid by means of combined serologic testing. Oral Surg Oral Med Oral Pathol Oral Radiol 2014; 117: 483–96.
Higgins TS, Cohen JC, Sinacori JT. Laryngeal mucous membrane pemphigoid: a systematic review and pooled-data analysis. Laryngoscope 2010; 120: 529–36.
Yasukochi A, Teye K, Ishii N, Hashimoto T. Clinical and immuno-logical studies of 332 Japanese patients tentatively diagnosed as anti-BP180-type mucous membrane pemphigoid: a novel BP180 C-terminal domain enzyme-linked immunosorbent assay. Acta Derm Venereol 2016; 96: 762–7.
Ishiko A, Shimizu H, Masunaga T, et al. 97-kDa linear IgA bullous dermatosis (LAD) antigen localizes to the lamina lucida of the epidermal basement membrane. J Invest Dermatol 1996; 106: 739–43.
Marinkovich MP, Taylor TB, Keene DR, Burgeson RE, Zone JJ. LAD-1, the linear IgA bullous dermatosis autoantigen, is a novel 120-kDa anchoring filament protein synthesized by epidermal cells. J Invest Dermatol 1996; 106: 734–8.
Syn WK, Ahmed MM. Esophageal involvement in cicatricial pemphigoid: a rare cause of dysphagia. Dis Esophagus 2004; 17: 180–2.
Furusawa K, Hasegawa T, Hirasawa Y, Ikeda S. Mucous membrane pemphigoid with esophageal stricture treated with balloon dilatation. J Dermatol 2015; 42: 325–7.
Zehou O, Raynaud JJ, Le Roux-Villet C, et al. Oesophageal involvement in 26 consecutive patients with mucous membrane pemphigoid. BrJ Dermatol 2017; 177: 1074–85.
Wozniak K, Gorkiewicz A, Olszewska M, et al. Cicatricial pemphigoid vegetans. IntJ Dermatol 2007; 46: 299–302.
Narbutt J, Cieptowska K, Drewnik A, Schwartz RA, Kowalewski C. Cicatricial pemphigoid vegetans in a Polish woman. Int J Dermatol 2015; 54: 317–9.
Jakubowska B, Kowalewski C, Ishii N, et al. Vegetating erosive cutaneous lesions and pyogenic granuloma in the course of mucous membrane pemphigoid: a case report and review of literature. Int Wound J 2018; 15: 909–13.
Letko E, Bhol K, Anzaar F, Perez VL, Ahmed AR, Foster CS. Chronic cicatrizing conjunctivitis in a patient with epidermolysis bullosa acquisita. Arch Ophthalmol 2006; 124: 1615–8.
Kim JH, Kim SC. Epidermolysis bullosa acquisita. J Eur Acad Dermatol Venereol 2013; 27: 1204–13.
Komorowski L, Müller R, Vorobyev A, et al. Sensitive and specific assays for routine serological diagnosis of epidermolysis bullosa acquisita. J Am Acad Dermatol 2013; 68: 89–95.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosures
Financial support: this work was supported by a grant from the National Centre of Science, Poland (no.NN402 661940). Conflicts of interest: none.
About this article
Cite this article
Wozniak, K., Jakubowska, B., Kalinska-Bienias, A. et al. Diagnosis of autoimmune subepidermal bullous diseases with mucous membrane involvement based on laser-scanning confocal microscopy. Eur J Dermatol 30, 516–523 (2020). https://doi.org/10.1684/ejd.2020.3765
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1684/ejd.2020.3765