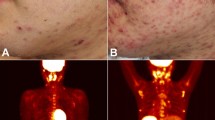
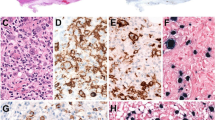
Abstract
Hydroa vacciniforme (HV) is a cutaneous subset of Epstein-Barr virus (EBV)-associated T/NK lymphoproliferative disorders (LPDs). Our previous case series study clearly showed a clinical spectrum of EBV-associated T/NK LPDs including HV, hypersensitivity to mosquito bites (HMB), chronic active EBV infection (CAEBV), and hemophagocytic lymphohistiocytosis (HLH). Patients with HV are divided into two groups: a benign subtype designated “classic HV” (cHV) and more serious systemic HV (sHV), also called “HV-like LPD” in the 2017 World Health Organization (WHO) classification. Patients with cHV usually have an increased number of EBV-infected γδT cells and patients with sHV without HMB are further classified into two groups: γδT-cell- and αβT-cell-dominant types. Patients with HMB, with or without HV-like eruptions, have an increased number of EBV-infected NK cells in the blood. Patients with cHV and γδT-cell-dominant sHV show a favourable prognosis, but the other subtypes such as αβT-cell-dominant sHV and HMB have a poor prognosis with mortality rates of 11.5 and 3.51 per 100 person-years, respectively. In addition to the clinical subtypes and the dominant lymphocyte subsets, the poor prognostic indicators include onset age over nine years and expression of the reactivation marker, BZLF1 mRNA. No prognostic correlation has been reported for anti-EBV antibody titres or EBV DNA load. The clinical subtypes and their prognostic factors should be considered for therapeutic interventions.
Similar content being viewed by others
References
Quintanilla-Martinez L, Ko YH, Kimura H, Jaffe ES. EBV-positive T-cell and NK-cell lymphoproliferative diseases of childhood. In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th Ed. Swerdlow SH, Campo E, Harris NL, et al. Lyon: WHO Press, 2017: 355–63.
Quintanilla-Martinez L, Kumar S, Fend F, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood 2000; 96: 443–51.
Cohen JI, Kimura H, Nakamura S, Ko Y-H, Jaffe ES. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts. Ann Oncol 2009; 20: 1472–82.
Iwatsuki K, Satoh M, Yamamoto T, et al. Pathogenic link between hydroa vacciniforme and Epstein-Barr virus-associated hematologic disorders. Arch Dermatol 2006; 142: 587–95.
Kimura H, Hoshino Y, Kanegane H, et al. Clinical and virologic characteristics of chronic active Epstein-Barr virus infection. Blood 2001; 98: 280–6.
Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in non-immunocompromised hosts: prospective analysis of 108 cases. Blood 2012; 119: 673–86.
Hirai Y, Yamamoto T, Kimura H, et al. Hydroa vacciniforme is associated with increased numbers of Epstein-Barr virus-infected γδT cells. J Invest Dermatol 2012; 132: 1401–8.
Kimura H, Miyake K, Yamauchi Y, et al. Identification of Epstein-Barr virus (EBV)-infected lymphocyte subtypes by flow cytometric in situ hybridization in EBV-associated lymphoproliferative diseases. J Infect Dis 2009; 200: 1078–87.
Tanaka C, Hasegawa M, Fujimoto M, et al. Phenotypic analysis in a case of hydroa vacciniforme-like eruptions associated with chronic active Epstein-Barr virus disease of γδ T cells. Br J Dermatol 2012; 166: 216–8.
Kimura H, Morishima T, Kanegane H, et al. Prognostic factors for chronic active Epstein-Barr virus infection. J Infect Dis 2003; 187: 527–33.
Ohshima K, Kimura H, Yoshino T, et al. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lympho-proliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Int 2008; 58: 209–17.
Miyake T, Yamamoto T, Hirai Y, et al. Survival rates and prognostic factors of Epstein-Barr virus-associated hydroa vacciniforme and hypersensitivity to mosquito bites. Br J Dermatol 2015; 172: 56–63.
Bazin PAE. Lecons théoriques et cliniques sur les affections génériques de la peau; tom 1, 1862: 599.
Yamamoto T, Hirai Y, Miyake T, Yamasaki O, Morizane S, Iwatsuki K. Oculomucosal and gastrointestinal involvement in Epstein-Barr virus-associated hydroa vacciniforme. Eur J Dermatol 2012; 22: 380–3.
Iwatsuki K, Xu Z, Takata M, et al. The association of latent Epstein-Barr virus infection with hydroa vacciniforme. Br J Dermatol 1999; 140: 715–21.
Morizane S, Suzuki D, Tsuji K, Oono T, Iwatsuki K. The role of CD4 and CD8 cytotoxic T lymphocytes in the formation of viral vesicles. Br J Dermatol 2005; 153: 981–6.
Oono T, Arata J, Masuda T, Ohtsuki Y. Coexistence of hydroa vacciniforme and malignant lymphoma. Arch Dermatol 1986; 122: 1306–9.
Tabata N, Aiba S, Ichinohazama R, et al. Hydroa vacciniforme-like lymphomatoid papulosis in a Japanese child: a new subset. J Am Acad Dermatol 1995; 32: 378–81.
Katagiri Y, Mitsuhashi Y, Kondo S, Kanazawa C, Iwatsuki K, Tsunoda T. Hydroa vacciniforme-like eruptions in a patient with chronic active EB virus infection. J Dermatol 2003; 30: 400–4.
Nitta Y, Iwatsuki K, Kimura H, et al. Fatal natural killer cell lymphoma arising in a patient with a crop of Epstein-Barr virus-associated disorders. EurJ Dermatol 2005; 15: 503–6.
Cho KH, Kim CW, Lee DY, Sohn SJ, Kim DW, Chung JH. An Epstein-Barr virus-associated lymphoproliferative lesion of the skin presenting as recurrent necrotic papulovesicles of the face. Br J Dermatol 1996; 134: 791–6.
Xu Z, Lian S. Epstein-Barr virus-associated hydroa vacciniforme-like cutaneous lymphoma in seven Chinese children. Pediatr Dermatol 2010; 27: 463–9.
Magana M, Sangueza P, Gil-Beristain J, et al. Angiocentric cutaneous T-cell lymphoma of childhood (hydroa-like lymphoma): a distinctive type of cutaneous T-cell lymphoma. J Am Acad Dermartol 1998; 38: 574–9.
Barrionuevo C, Anderson VM, Zevallos-Giampietri E, et al. Hydroa-like cutaneous T-cell lymphoma: a clinicopathologic and molecular genetic study of 16 pediatric cases from Peru. Appl Immunohistochem Mol Morphol 2002; 10: 7–14.
Chen HH, Hsiao CH, Chiu HC. Hydroa vacciniforme-like primary cutaneous CD8-positive T-cell lymphoma. Br J Dermatol 2002; 147: 587–91.
Cho KH, Lee SH, Kim CW, et al. Epstein-Barr virus-associated lymphoproliferative lesions presenting as a hydroa vacciniforme-like eruption: an analysis of six cases. Br J Dermatol 2004; 151: 372–80.
Doeden K, Molina-Kirsch H, Perez E, Warnke R, Sundram U. Hydroa-like lymphoma with CD56 expression. J Cutan Pathol 2008; 35: 488–94.
Quintanilla-Martinez L, Ridaura C, Nagi F, et al. Hydroa vacciniforme-like lymphoma: a chronic EBV+ lymphoproliferative disorder with risk to develop a systemic lymphoma. Blood 2013; 122: 3101–10.
Asada H, Saito-Katsuragi M, Niizeki H, et al. Mosquito salivary gland extracts induce EBV-infected NK cell oncogenesis via CD4+ T cells in patients with hypersensitivity to mosquito bites. J Invest Dermatol 2005; 125: 956–61.
Tokura Y, Ishihara S, Tagawa S, Seo N, Ohshima K, Takigawa M. Hypersensitivity to mosquito bites as the primary clinical manifestation of a juvenile type of Epstein-Barr virus-associated natural killer cell leukemia/lymphoma. J Am Acad Dermatol 2001; 45: 569–78.
Nomura H, Egami S, Kasai H, et al. An elderly patient with chronic active Epstein-Barr virus infection with severe hydroa vacciniforme-like eruptions associated with αβT-cell proliferation. J Dermatol 2014; 41: 360–2.
Arai A, Imadome K, Watanabe Y, et al. Clinical features of adult-onset chronic active Epstein-Barr virus infection: a retrospective analysis. Int J Hematol 2011; 93: 602–9.
Ohga S, Ishimura M, Yoshimoto G, et al. Clonal origin of Epstein-Barr virus (EBV)-infected T/NK-cell subpopulations in EBV-positive T/NK-cell lymphoproliferative disorders of childhood. J Clin Virol 2011; 51: 31–7.
Ito Y, Suzuki R, Torii Y, et al. HLA-A*26 and HLA-B*52 are associated with a risk of developing EBV-associated T/NK lymphoproliferative disease. Blood 2013, Available at: http://www.bloodjournal.org/content/early/2011/11/16/blood-2011-10-381921/tab-e-letters.
Huang Y, de Reynies A, de Leval L, et al. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood 2010; 115: 1226–37.
Karube K, Nakagawa M, Tsuzuki S, et al. Identification of FOXO3 and PRDM1 as tumor-suppressor gene candidates in NK-cell neoplasms by genomic and functional analysis. Blood 2011; 118: 3195–204.
Jiang L, Gu ZH, Yan ZX, et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat Genet 2015; 47: 1061–6.
Cohen JI, Dropulic L, Hsu AP, et al. Association of GATA2 deficiency with severe primary Epstein-Barr virus (EBV) infection and EBV-associated cancers. Clin Infect Dis 2016; 63: 41–7.
Yamamoto T, Hirai Y, Miyake T, et al. Epstein-Barr virus reactivation is induced, but abortive, in cutaneous lesions of systemic hydroa vacciniforme and hypersensitivity to mosquito bites. J Dermatol Sci 2016; 82: 153–9.
Suzuki D, Tsuji K, Yamamoto T, Fujii K, Iwatsuki K. Production of proinflammatory cytokines without invocation of cytotoxic effects by an Epstein-Barr virus-infected natural killer cell line established from a patient with hypersensitivity to mosquito bites. Exp Hematol 2010; 38: 933–44.
Paik JH, Choe JY, Kim H, et al. Clinicopathological categorization of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disease: an analysis of 42 cases with an emphasis on prognostic implications. Leuk Lymphoma 2017; 58: 53–63.
Kawa K, Sawada A, Sato M, et al. Excellent outcome of allogenic hematopoietic SCT with reduced-intensity conditioning for the treatment of chronic active EBV infection. Bone Marrow Transplant 2011; 46: 77–83.
Gotoh K, Ito Y, Shibata-Watanabe Y, et al. Clinical and virological characteristics of 15 patients with chronic active Epstein-Barr virus infection treated with hematopoietic stem cell transplantation. Clin Infect Dis 2008; 46: 1525–34.
Sato E, Ohga S, Kuroda H, et al. Allogenic hematopoietic stem cell transplantation for Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disease in Japan. Am J Hematol 2008; 83: 721–7.
Toksoy A, Strifler S, Benoit S, et al. Hydroa vacciniforme-like skin lesions in Epstein-Barr-virus-associated T-cell lymphoproliferation with subsequent development of aggressive NK/T-cell lymphoma. Acta Derm Venereol 2017; 97: 379–80.
Koyama M, Takeshita Y, Sakata A, et al. Cytotoxic chemotherapy successfully induces durable complete remission in 2 patients with mosquito allergy resulting from Epstein-Barr virus-associated T-/natural killer cell lymphoproliferative disease. Int J Hematol 2005; 82: 437–40.
Beltrán BE, Msaza I, Moisés-Alfaro CB, et al. Thalidomide for the treatment of hydroa vacciniforme-like lymphoma: report of four pediatric cases from Peru. Am J Hematol 2014; 89: 1160–1.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Iwatsuki, K., Miyake, T., Hirai, Y. et al. Hydroa vacciniforme: a distinctive form of Epstein-Barr virus-associated T-cell lymphoproliferative disorders. Eur J Dermatol 29, 21–28 (2019). https://doi.org/10.1684/ejd.2018.3490
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1684/ejd.2018.3490