Abstract
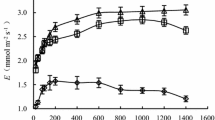
Wetlands are the intermediate between terrestrial and open water systems, and are thus fundamentally tied to changes in water table. Under normal climate conditions, wetlands may experience short periods of water scarcity through seasonal variations in precipitation or surface water run-off. However, during anomalous drought episodes, wetland plants may experience elevated water stress resulting in considerable decreases of both productivity and survival. Despite the intricate relationship between wetland macrophytes and water supply, little is known about the physiological responses of these plants to short-term water deficits. Therefore, the purpose of this study was to evaluate the effects of simulated drought on plant water relations in five herbaceous wetland species (monocots Carex alata, Juncus effusus, Peltandra virginica, and dicots Saururus cernuus, Justicia americana). In general, plant response to water deprivation may include drought avoidance (e.g., changes in stomatal conductance, leaf area, and leaf orientation) and/or drought tolerance (maintaining cell turgor through osmotic adjustments or cell wall elasticity). In this study, simulated drought resulted in significant decreases in xylem water potential (Ψxylem) for all five species, suggesting that these plants were physiologically affected by water deficit. Four of the five species showed outward signs of drought avoidance, including significant reductions in transpiration (C. alata, P. virginica, J. americana, and S. cernuus) and decreases in leaf area (P. virginica and J. americana). Interestingly, while adjustments in transpiration were observed for most plants during the dry period, no significant changes in water use efficiencies (WUE) were detected until after water repletion. Notably, two species (C. alata and P. virginica) had enhanced WUE as water availability returned to pre-drought conditions. Water deficits also promoted drought tolerance responses in all five species, as indicated by a change in bulk modulus of elasticity (∈; all species) and decreased osmotic potential (Ψπ; P. virginica). Taken as a whole, this study reveals two contrasting drought tolerance strategies in wetland herbs. While four of the species alter ∈ to generate declines in Ψ, P. virginica favored decreases in osmotic potential (as indicated by decreases in Ψπ at full saturation and at turgor loss point).
Similar content being viewed by others
Literature Cited
Amlin, N. M. and S. B. Rood. 2002. Comparative tolerances of riparian willows and cottonwoods to water table decline. Wetlands 22: 338–46.
Ayoub, A., M. Khalil, and J. Grace. 1992. Acclimation to drought in Acer pseudoplatanus L. (sycamore) seedlings. Journal of Experimental Botany 43: 1591–1602.
Barr, H. D. and P. E. Weatherley. 1962. A re-examination of the relative turgidity technique of estimating water deficit in leaves. Australian Journal of Biological Sciences 15: 413–28.
Black, C. A. 1965. Methods of Soil Analysis: Part I. Physical and Mineralogical Properties. American Society of Agronomy, Madison, Wisconsin, USA.
Blake, T. J., E. Bevilacqua, and J. J. Zwiazek. 1991. Effects of repeated stress on turgor pressure and cell elasticity changes in black spruce seedlings. Canadian Journal of Forest Research 21: 1329–33.
Bray, E. A. 1993. Molecular responses to water deficits. Plant Physiology 103: 1035–40.
Brock, M. T. and C. Galen. 2005. Drought tolerance in the alpine dandelion, Taraxacum ceratophorum (Asteraceae), its exotic congerner T. officinale, and interspecific hybrids under natural and experimental conditions. American Journal of Botany 92: 1311–21.
Bush, D. E., W. F. Loftus, and O. L. Bass. 1998. Long-term hydrolic effects on marsh plant community structure in the southern everglades. Wetlands 18: 203–41.
Chen, X. M., G. B. Begonia, D. M. Alm, and J. D. Hesketh. 1993. Responses of soybean leaf photosynthesis to CO2 and drought. Photosynthetica 29: 447–54.
Clifford, S. C., S. K. Arndt, J. E. Corlett, S. Joshi, N. Sankhla, M. Popp, and H. G. Jones. 1998. The role of solute accumulation, osmotic adjustment and changes in cell wall elasticity in drought tolerance in Ziziphus mauritiana (Lamk.). Journal of Experimental Botany 49: 967–77.
Corcuera, L., J. J. Camarero, and E. Gil-Pelegrín. 2002. Functional groups in Quercus species derived from the analysis of pressure-volume curves. Trees 16: 465–72.
Cowardin, L. M., V. Carter, F. C. Golet, and E. T. LaRoe. 1979. Classification of Wetlands and Deepwater Habitats of the United States. U.S. Government Printing Office, Washington, DC, USA.
Davies, F. S. and A. N. Lakso. 1979. Diurnal and seasonal changes in leaf water potential components and elastic properties in response to water stress in apple trees. Physiologia Plantarum 46: 109–14.
Dichio, B., C. Xiloyannis, K. Angelopoulos, V. Nuzzo, S. A. Bufo, and G. Celano. 2003. Drought-induced variations of water relations parameters in Olea europaea. Plant and Soil 257: 381–89.
Ferris, R., M. Sabatti, F. Miglietta, R. F. Mills, and G. Taylor. 2001. Leaf area is stimulated in Populus by free air CO2 enrichment (POPFACE), through increased cell expansion and production. Plant, Cell and Environment 24: 305–15.
Gaiser, T., I. de Barros, F. M. Lange, and J. R. Williams. 2004. Water use efficiency of a maize/cowpea intercrop on a highly acidic tropical soil as affected by liming and fertilizer application. Plant and Soil 263: 165–71.
Gao, X., C. Zou, L. Wang, and F. Zhang. 2004. Silicon improves water use efficiency in maize plants. Journal of Plant Nutrition 27: 1457–70.
Girma, F. S. and D. R. Krieg. 1992. Osmotic adjustment in Sorghum. I. mechanisms of diurnal osmotic potential changes. Plant Physiology 99: 577–82.
Grammatikopoulos, G. 1999. Mechanism of drought tolerance in two Mediterranean seasonal dimorphic shrubs Australian Journal of Plant Physiology 26: 587–93.
Holbrook, N. M. and F. E. Putz. 1996. From epiphyte to tree: differences in leaf structure and leaf water relation associated with the transition in growth form in eight species of hemiepiphytes. Plant Cell and Environment 19: 631–42.
Huang, X. M., H. B. Huang, and F. F. Gao. 2000. The growth potential generated in citrus fruit under water stress and its relevant mechanism. Scientia Horticulturae 83: 227–40.
Joly, R. J. 1985. Techniques for determining seedling water status and their effectiveness in assessing stress. p. 17–28. In M. L. Duryea (ed.) Evaluating Seedling Quality: Principles, Procedures, and Predictive Abilities of Major Tests. Forest Research Laboratory, Oregon State University, Corvallis, OR, USA.
Jones, H. G. and J. E. Corlett. 1992. Current topics in drought physiology. Journal of Agricultural Science 119: 291–96.
Karamanos, A. J. 1984. Way of detecting adaptive responses of cultivated plants to drought: an agronomic approach. p. 91–101. In N. S. Margaris, M. Arianoutsou-Farragitakiand, and W. C. Oechel (eds.) Being Alive on Land: Tasks for Vegetation Science. W. Junk Publishers, The Hague, The Netherlands.
Kercher, S. M. and J. B. Zedler. 2004. Flood tolerance in wetland angiosperms: a comparison of invasive and noninvasive species. Aquatic Botany 80: 89–102.
Koide, R. T., R. H. Robichaux, S. R. Morse, and C. M. Smith. 1989. Plant water status, hydraulic resistance and capacitance. p. 161–78. In R. W. Pearcy, J. Ehleringer, H. A. Mooney, and P. W. Rundel (eds.) Plant Physiological Ecology: Field Methods and Instrumentation. Chapman and Hall, New York, NY, USA.
Kozlowski, T. T., P. J. Kramer, and S. G. Pallardy. 1990. The Physiological Ecology of Woody Plants. Academic Press, New York, NY, USA.
Kramer, P. J. 1983. Water Relations of Plants. Academic Press, New York, NY, USA.
Kramer, P. J. and J. S. Boyer. 1995. Water Relations of Plants and Soils. Academic Press, New York, NY, USA.
Liu, F., M. N. Andersen, S. E. Jacobsen, and C. R. Jensen. 2005. Stomatal control and water use efficiency of soybean (Glycine max L. Merr.) during progressive soil drying. Environmental and Experimental Botany 54: 33–40.
Lo Gullo, M. A., S. Salleo, and R. Rosso. 1986. Drought avoidance strategy in Ceratonia siliqua L. a mesomorphic-leaved tree in the xeric Mediterranean area. Annals of Botany 58: 745–56.
Marron, N., D. Delay, J. M. Petit, E. Dreyer, G. Kahlem, F. M. Delmotte, and F. Brignolas. 2002. Physiological traits of two Populus × euramericana clones, Luisa Avanzo and Dorskamp, during a water stress and re-watering cycle. Tree Physiology 22: 849–58.
Meinzer, F. C., M. J. Clearwater, and G. Goldstein. 2001. Water transport in trees: current perspectives, new insights and some controversies. Environmental and Experimental Botany 45: 239–62.
Meinzer, F. C., D. A. Grantz, G. Goldstein, and N. Z. Saliendra. 1990. Leaf water relations and maintenance of gas exchange in coffee cultivars grown in drying soil. Plant Physiology 94: 1781–87.
Mitsch, W. J., C. L. Dorge, and J. R. Wiemhoff. 1979. Ecosystem dynamics and a phosphorus budget of an alluvial cypress swamp in southern Illinois. Ecology 60: 1116–24.
Mitsch, W. J. and J. G. Gosselink. 1986. Wetlands. Van Nostrand Reinhold Co., New York, NY, USA.
Morgan, J. M. 1984. Osmoregulation and water stress in higher plants. Annual Reviews in Plant Physiology 35: 299–319.
Mulhouse, J. M., L. E. Burbage, and R. R. Sharitz. 2005. Seed bank-vegetation relations in herbaceous Carolina bays: responses to climate variability. Wetlands 25: 738–47.
Munns, R. 1988. Why measure osmotic adjustment? Australian Journal of Plant Physiology 15: 717–26.
O’Neal, M. E., D. A. Landis, and R. Isaacs. 2002. An inexpensive, accurate method for measuring leaf area and defoliation through digital image analysis. Journal of Economic Entomology 95: 1190–94.
Pavlik, B. M. 1984. Seasonal changes of osmotic pressure, symplasmic water content and tissue elasticity in the blades of dune grasses growing in situ along the coast of Oregon. Plant, Cell and Environment 7: 531–39.
Peltier, J. P. and G. Marigo. 1999. Drought adaptation in Fraxinus excelsior L.: physiological basis of the elastic adjustment. Journal of Plant Physiology 154: 529–35.
Reichstein, M., J. D. Tenhunen, O. Roupsard, J. M. Ourcival, S. Rambal, F. Miglietta, A. Peressoti, M. Pecchiaris, G. Tirone, and R. Valentini. 2002. Severe drought effects on ecosystem CO2 and H2O fluxes at three Mediterranean evergreen sites, revision of current hypotheses? Global Change Biology 8: 999–1017.
Saito, T. and I. Terashima. 2004. Reversible decreases in the bulk elastic modulus of mature leaves of deciduous Quercus species subjected to two drought treatments. Plant, Cell and Environment 27: 863–75.
Savé, R., J. Peñuelas, O. Marfá, and L. Serrano. 1993. Changes in leaf osmotic and elastic properties and canopy structure of strawberries under mild water stress. Horticultural Science 28: 925–27.
Scholander, P. F., H. T. Hammel, E. D. Bradstreet, and E. A. Hemmingsen. 1965. Sap pressure in vascular plants. Science 148: 339–46.
Schulte, P. J. 1992. The units of currency for plant water status. Plant, Cell and Environment 15: 7–10.
Schultz, H. R. and M. A. Matthews. 1993. Xylem development and hydraulic conductance in sun and shade shoots of grapevine (Vitis vinifera L.): evidence that low light uncouples water transport capacity from leaf area. Planta 190: 393–406.
Serrano, L. and J. Penuelas. 2005. Contribution of physiological and morphological adjustments to drought resistance in two Mediterranean tree species. Biologia Plantarum 49: 551–59.
Slavík, B. 1974. Methods of Studying Plant Water Relations. Springer-Verlag, Berlin, Germany.
Touchette, B. W. 2006. Salt tolerance in a Juncus roemerianus brackish marsh: spatial variations in plant water relations. Journal of Experimental Marine Biology and Ecology 337: 1–12.
Tschaplinski, T. J., G. A. Tuskan, G. M. Gebre, and D. E. Todd. 1998. Drought resistance of two hybrid Populus clones grown in a large-scale plantation. Tree Physiology 18: 653–58.
Turner, N. C., R. A. Spurway, and E. D. Schulze. 1984. Comparison of water potentials measured by in-situ psychrometry and pressure chamber in morphologically different species. Plant Physiology 74: 316–19.
Tyree, M. T. and H. T. Hammel. 1972. The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. Journal of Experimental Botany 23: 267–82.
White, D. A., N. C. Turner, and J. H. Galbraith. 2000. Leaf water relations and stomatal behavior of four allopatric Eucalyptus species planted in Mediterranean southwestern Australia. Tree Physiology 20: 1157–65.
White, R. H., M. C. Engelke, S. J. Anderson, B. A. Ruemmele, K. B. Marcum, and G. R. Taylor. 2001. Zoysiagrass water relations. Crop Science 41: 133–38.
Wilcox, D. A., J. E. Meeker, P. L. Hudson, B. J. Armitage, M. G. Black, and D. G. Uzarski. 2002. Hydrologic variability and the application of index of biotic integrity metrics to wetlands: a Great Lakes evaluation. Wetlands 22: 588–615.
Wilson, J. R., M. M. Ludlow, M. J. Fisher, and E. D. Schulze. 1980. Adaptation to water stress of the leaf water relations of four tropical forage species. Australian Journal of Plant Physiology 7: 207–20.
Yin, C., X. Wang, B. Duan, J. Luo, and C. Li. 2005. Early growth, dry matter allocation and water use efficiency of two sympatric Populus species as affected by water stress. Environmental and Experimental Botany 53: 315–22.
Zlatev, Z. S. 2005. Effects of water stress on leaf water relations of young bean plants. Journal of Central European Agriculture 6: 5–14.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Touchette, B.W., Iannacone, L.R., Turner, G.E. et al. Drought tolerance versus drought avoidance: A comparison of plant-water relations in herbaceous wetland plants subjected to water withdrawal and repletion. Wetlands 27, 656–667 (2007). https://doi.org/10.1672/0277-5212(2007)27[656:DTVDAA]2.0.CO;2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1672/0277-5212(2007)27[656:DTVDAA]2.0.CO;2