Abstract
Objectives
In this study, we explored how adiponectin mediated urotensin II (UII)-induced tumor necrosis factor-α (TNF-α) and α-smooth muscle actin (α-SMA) expression and ensuing intracellular signaling pathways in adventitial fibroblasts (AFs).
Methods
Growth-arrested AFs and rat tunica adventitia of vessels were incubated with UII and inhibitors of signal transduction pathways for 1–24 h. The cells were then harvested for TNF-α receptor (TNF-α-R) messenger RNA (mRNA) and TNF-α protein expression determination by reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA), respectively. Adiponectin and adiponectin receptor (adipoR) expression was measured by RT-PCR, quantitative real-time PCR (qPCR), immunohistochemical analysis, and cell counting kit-8 (CCK-8) cell proliferation experiments. We then quantified TNF-α and α-SMA mRNA and protein expression levels by qPCR and immunofluorescence (IF) staining. RNA interference (RNAi) was used to explore the function of the adipoR genes. To investigate the signaling pathway, we applied western blotting (WB) to examine phosphorylation of adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK). In vivo, an adiponectin (APN)-knockout (APN-KO) mouse model mimicking adventitial inflammation was generated to measure TNF-α and α-SMA expression by application of qPCR and IF, with the goal of gaining a comprehensive atlas of adiponectin in vascular remodeling.
Results
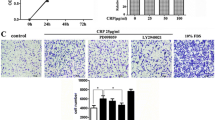
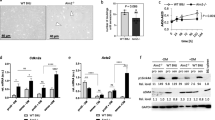
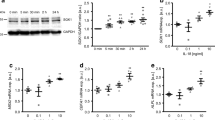
In both cells and tissues, UII promoted TNF-α protein and TNF-α-R secretion in a dose- and time-dependent manner via Rho/protein kinase C (PKC) pathway. We detected marked expression of adipoR1, T-cadherin, and calreticulin as well as a moderate presence of adipoR2 in AFs, while no adiponectin was observed. Globular adiponectin (gAd) fostered the growth of AFs, and acted in concert with UII to induce α-SMA and TNF-α through the adipoR1/T-cadherin/calreticulin/AMPK pathway. In AFs, gAd and UII synergistically induced AMPK phosphorylation. In the adventitial inflammation model, APN deficiency up-regulated the expression of α-SMA, UII receptor (UT), and UII while inhibiting TNF-α expression.
Conclusions
From the results of our study, we can speculate that UII induces TNF-α protein and TNF-α-R secretion in AFs and rat tunica adventitia of vessels via the Rho and PKC signal transduction pathways. Thus, it is plausible that adiponectin is a major player in adventitial progression and could serve as a novel therapeutic target for cardiovascular disease administration.
概要
背景
血管外膜炎症在血管重构的发生发展过程中起着非常重要的作用。已有众多研究提示尾加压素II(UII)和脂联素(APN)在调节细胞免疫、代谢、炎症和凋亡方面具有作用。然而,关于它们对血管外膜重构的重要指示因子α-平滑肌肌动蛋白(α-SMA)和肿瘤坏死因子α(TNF-α)表达的影响所知有限。
目的
探索APN如何介导UII诱导的血管外膜分泌TNF-α和α-SMA及其信号转导通路机制。
方法
在体外实验中,将原代培养的大鼠血管外膜成纤维细胞(AFs)和血管外膜组织,用UII、APN和各种信号通路阻断剂孵育1~24小时。收集细胞和组织,分别采用RT-PCR和ELISA检测TNF-α及其受体mRNA和蛋白表达。采用RT-PCR、qPCR、免疫组化、CCK-8等检测APN及其受体(adipoR)的表达。然后通过qPCR和免疫荧光染色(IF)检测TNF-α和α-SMA蛋白和mRNA表达水平。采用RNA干扰(RNAi)研究adipoR基因的功能。应用western blotting观察AMPK磷酸化情况来研究信号通路。在体内实验中,建立APN基因敲除小鼠颈动脉外膜炎症模型,应用qPCR和IF检测TNF-α和α-SMA的表达。
结果
在细胞和组织中,UII通过Rho/PKC途径以剂量和时间依赖性的方式促进TNF-α及其受体的表达。adipoR1、T-cadherin和calreticulin在AFs中高表达,adipoR2低表达,没有观察到APN在AFs中表达。gAd与UII通过adipoR1/T-cadherin/calreticulin/AMPK途径协同而非抑制AFs中α-SMA和TNF-α的表达。同时,gAd和UII能够协同诱导AMPK磷酸化。在APN基因敲除小鼠构建的颈动脉外膜炎症模型中,APN的缺失可上调α-SMA、UII受体(UT)和UII的表达,而抑制TNF-α的表达。
结论
根据我们的研究结果,可以推测UII通过Rho和PKC信号转导通路诱导AFs和大鼠血管外膜中TNF-α及其受体的分泌。APN在血管外膜炎症性疾病的发生发展过程中扮演了重要角色,并可能成为心血管疾病治疗的新靶点。
Similar content being viewed by others
References
Ames RS, Sarau HM, Chambers JK, et al., 1999. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature, 401(6750):282–286. https://doi.org/10.1038/45809
Avagimyan A, Kajaia A, Gabunia L, et al., 2022. Urotensin-II as a promising key-point of cardiovascular disturbances sequel. Curr Probl Cardiol, 47(11):101074. https://doi.org/10.1016/j.cpcardiol.2021.101074
Bobbert P, Antoniak S, Schultheiss HP, et al., 2008. Globular adiponectin but not full-length adiponectin induces increased procoagulability in human endothelial cells. J Mol Cell Cardiol, 44(2):388–394. https://doi.org/10.1016/j.yjmcc.2007.10.018
Dong X, Ye XJ, Song NN, et al., 2013. Urotensin II promotes the production of LTC4 in rat aortic adventitial fibroblasts through NF-κB-5-LO pathway by p38 MAPK and ERK activations. Heart Vessels, 28(4):514–523. https://doi.org/10.1007/s00380-012-0291-0
Enzerink A, Vaheri A, 2011. Fibroblast activation in vascular inflammation. J Thromb Haemost, 9(4):619–626. https://doi.org/10.1111/j.1538-7836.2011.04209.x
Evans BR, Yerly A, van der Vorst EPC, et al., 2022. Inflammatory mediators in atherosclerotic vascular remodeling. Front Cardiovasc Med, 9:868934. https://doi.org/10.3389/fcvm.2022.868934
Hassan GS, Douglas SA, Ohlstein EH, et al., 2005. Expression of urotensin-II in human coronary atherosclerosis. Peptides, 26(12):2464–2472. https://doi.org/10.1016/j.peptides.2005.05.028
Lau WB, Ohashi K, Wang YJ, et al., 2017. Role of adipokines in cardiovascular disease. Circ J, 81(7):920–928. https://doi.org/10.1253/circj.CJ-17-0458
Li AC, Glass CK, 2002. The macrophage foam cell as a target for therapeutic intervention. Nat Med, 8(11):1235–1242. https://doi.org/10.1038/nm1102-1235
Liu LH, Shi ZH, Ji XH, et al., 2022. Adipokines, adiposity, and atherosclerosis. Cell Mol Life Sci, 79(5):272. https://doi.org/10.1007/s00018-022-04286-2
Lu D, Peng F, Li J, et al., 2019. Urotensin II promotes secretion of LTB4 through 5-lipoxygenase via the UT-ROS-Akt pathway in RAW264.7 macrophages. Arch Med Sci, 15(4):1065–1072. https://doi.org/10.5114/aoms.2019.85197
Nosalski R, Guzik TJ, 2017. Perivascular adipose tissue inflammation in vascular disease. Br J Pharmacol, 174(20):3496–3513. https://doi.org/10.1111/bph.13705
Park SL, Lee BK, Kim YA, et al., 2013. Inhibitory effect of an urotensin II receptor antagonist on proinflammatory activation induced by urotensin II in human vascular endothelial cells. Biomol Ther (Seoul), 21(4):277–283. https://doi.org/10.4062/biomolther.2013.051
Pereira-Castro J, Brás-Silva C, Fontes-Sousa AP, 2019. Novel insights into the role of urotensin II in cardiovascular disease. Drug Discov Today, 24(11):2170–2180. https://doi.org/10.1016/j.drudis.2019.08.005
Rami AZA, Hamid AA, Anuar NNM, et al., 2022. Exploring the relationship of perivascular adipose tissue inflammation and the development of vascular pathologies. Mediators Inflamm, 2022:2734321. https://doi.org/10.1155/2022/2734321
Ruan H, Dong LQ, 2016. Adiponectin signaling and function in insulin target tissues. J Mol Cell Biol, 8(2):101–109. https://doi.org/10.1093/jmcb/mjw014
Sowka A, Dobrzyn P, 2021. Role of perivascular adipose tissue-derived adiponectin in vascular homeostasis. Cells, 10(6):1485. https://doi.org/10.3390/cells10061485
Stenmark KR, Yeager ME, el Kasmi KC, et al., 2013. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol, 75:23–47. https://doi.org/10.1146/annurev-physiol-030212-183802
Tinajero MG, Gotlieb AI, 2020. Recent developments in vascular adventitial pathobiology: the dynamic adventitia as a complex regulator of vascular disease. Am J Pathol, 190(3):520–534. https://doi.org/10.1016/j.ajpath.2019.10.021
von der Thüsen JH, van Berkel TJC, Biessen EAL, 2001. Induction of rapid atherogenesis by perivascular carotid collar placement in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Circulation, 103(8):1164–1170. https://doi.org/10.1161/01.CIR.103.8.1164
Wang ZV, Scherer PE, 2016. Adiponectin, the past two decades. J Mol Cell Biol, 8(2):93–100. https://doi.org/10.1093/jmcb/mjw011
Watson AMD, Olukman M, Koulis C, et al., 2013. Urotensin II receptor antagonism confers vasoprotective effects in diabetes associated atherosclerosis: studies in humans and in a mouse model of diabetes. Diabetologia, 56(5):1155–1165. https://doi.org/10.1007/s00125-013-2837-9
Yamauchi T, Kamon J, Ito Y, et al., 2003. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature, 423(6941):762–769. https://doi.org/10.1038/nature01705
Zhang R, Wu J, Liu D, et al., 2013. Anti-inflammatory effect of full-length adiponectin and proinflammatory effect of globular adiponectin in esophageal adenocarcinoma cells. Oncol Res, 21(1):15–21. https://doi.org/10.3727/096504013X13786659070235
Zhang YG, Li YG, Wei RH, et al., 2008a. Urotensin II is an autocrine/paracrine growth factor for aortic adventitia of rat. Regul Pept, 151(1–3):88–94. https://doi.org/10.1016/j.regpep.2008.10.004
Zhang YG, Li J, Li YG, et al., 2008b. Urotensin II induces phenotypic differentiation, migration, and collagen synthesis of adventitial fibroblasts from rat aorta. J Hypertens, 26(6):1119–1126. https://doi.org/10.1097/HJH.0b013e3282fa1412
Zhang YG, Hu YC, Mao YY, et al., 2010. Transforming growth factor-β1 involved in urotensin II-induced phenotypic differentiation of adventitial fibroblasts from rat aorta. Chin Med J (Engl), 123(24):3634–3639. https://doi.org/10.3760/cma.j.issn.0366-6999.2010.24.023
Zhang YG, Bao SL, Kuang ZJ, et al., 2014. Urotensin II promotes monocyte chemoattractant protein-1 expression in aortic adventitial fibroblasts of rat. Chin Med J (Engl), 127(10):1907–1912. https://doi.org/10.3760/cma.j.issn.0366-6999.20132795
Zhao K, Yang CX, Li P, et al., 2020. Epigenetic role of N6-methyladenosine (m6A) RNA methylation in the cardiovascular system. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 21(7):509–523. https://doi.org/10.1631/jzus.B1900680
Zhao K, Zhang J, Xu TH, et al., 2021. Low-intensity pulsed ultrasound ameliorates angiotensin II-induced cardiac fibrosis by alleviating inflammation via a caveolin-1-dependent pathway. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 22(10):818–838. https://doi.org/10.1631/jzus.B2100130
Zhou X, Li JQ, Wei LJ, et al., 2020. Silencing of DsbA-L gene impairs the PPARγ agonist function of improving insulin resistance in a high-glucose cell model. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 21(12):990–998. https://doi.org/10.1631/jzus.B2000432
Acknowledgments
This work was supported by the the National Natural Science Foundation of China (No. 82003372), the Natural Science Foundation of Hubei Province (Nos. 2018CFB747 and 2018CFB537), and the Educational Commission of Hubei Province (Nos. B2017112 and B20181130), China. We are grateful to Yongfeng QI, Liling WU, and Chaoshu TANG (School of Basic Medical Sciences, Peking University, Beijing, China), Dingfang BU, Dan LU, Fen PEN, and Yongyan HU (Peking University First Hospital, Beijing, China) for their helpful suggestions.
Author information
Authors and Affiliations
Contributions
Jun LI and Limin LUO completed all the experimental operations and drafted the article. Xiao DONG was responsible for the culture of cells and the feeding of animals. Yonggang ZHANG conducted statistics and analysis of experimental data. Shuyi DANG and Xiaogang GUO took part in drafting the article or revising it critically for important intellectual content. Wenhui DING contributed to conception and design. All authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Corresponding author
Additional information
Compliance with ethics guidelines
Jun LI, Limin LUO, Yonggang ZHANG, Xiao DONG, Shuyi DANG, Xiaogang GUO, and Wenhui DING declare that they have no conflict of interest.
The study design was carried out under the approval of the Committee on the Ethics of Animal Experiments of Peking University First Hospital (No. J201166). All animal surgeries were performed under sodium pentobarbital anesthesia and efforts were made to alleviate suffering. All institutional and national guidelines for the care and use of laboratory animals were followed.
Supplementary information
Tables S1–S3
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Li, J., Luo, L., Zhang, Y. et al. Globular adiponectin-mediated vascular remodeling by affecting the secretion of adventitial-derived tumor necrosis factor-α induced by urotensin II. J. Zhejiang Univ. Sci. B 23, 1014–1027 (2022). https://doi.org/10.1631/jzus.B2200346
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B2200346
Key words
- Urotensin II
- Adiponectin
- Signal transduction
- Adventitial fibroblast
- RNA interference (RNAi)
- Adiponectin-knockout (APN-KO)