Abstract
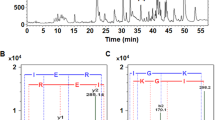
The aim of this work is to discover the inhibitory mechanism of tea peptides and to analyse the affinities between the peptides and the angiotensin-converting enzyme (ACE) as well as the stability of the complexes using in vitro and in silico methods. Four peptide sequences identified from tea, namely peptides I, II, III, and IV, were used to examine ACE inhibition and kinetics. The half maximal inhibitory concentration (IC50) values of the four peptides were (210.03±18.29), (178.91±5.18), (196.31±2.87), and (121.11±3.38) µmol/L, respectively. The results of Lineweaver-Burk plots showed that peptides I, II, and IV inhibited ACE activity in an uncompetitive manner, which requires the presence of substrate. Peptide III inhibited ACE in a non-competitive manner, for which the presence of substrate is not necessary. The docking simulations showed that the four peptides did not bind to the active sites of ACE, indicating that the four peptides are allosteric inhibitors. The binding free energies calculated from molecular dynamic (MD) simulation were −72.47, −42.20, −52.10, and −67.14 kcal/mol (1 kcal=4.186 kJ), respectively. The lower IC50 value of peptide IV may be attributed to its stability when docking with ACE and changes in the flexibility and unfolding of ACE. These four bioactive peptides with ACE inhibitory ability can be incorporated into novel functional ingredients of black tea.
摘要
目的
探究红茶中分离的茶叶多肽与血管紧张素转化酶(ACE)的结合关系以及结合稳定性, 从而发现红茶多肽对ACE的抑制机理.
创新点
ACE是哺乳动物调节血压的关键酶, 越来越多的研究着力于从食物蛋白中发现天然无副作用的ACE抑制剂. 计算机模拟技术具有高效、低成本、高通量等优点, 本研究从体外实验和计算机模拟两个维度来分析多肽与ACE之间的相互作用, 并使用了分子对接和分子动力学模拟两种计算机模拟方法, 使结果更加可靠.
方法
用体外酶抑制实验获取茶叶多肽(红茶中分离)对ACE的半抑制浓度(IC50);用酶动力学实验探究红茶多肽对ACE的抑制类型;通过分子对接技术预测茶叶多肽在ACE蛋白中的结合位置;通过100 ns的分子动力学模拟实验评价各多肽对ACE蛋白自由度和展开度的影响以及多肽-蛋白结合物的稳定性.
结论
四条茶叶多肽都表现出较高的体外ACE抑制活性(IC50值分别为(210.03±18.29)、(178.91±5.18)、(196.31±2.87)和(121.11±3.38) µmol/L), 酶动力学实验表明多肽I、II和IV对ACE的抑制类型为反竞争性抑制(即需要底物先与酶结合才能发挥作用), 多肽III为非竞争性抑制(即不管底物是否与酶结合都能发挥作用). 分子对接结果显示四条多肽都对接在远离ACE活性口袋的空腔中, 分子动力学模拟结果揭示多肽的ACE抑制能力与其对ACE蛋白残基自由度和蛋白展开度的影响存在相关性, 多肽IV体外抑制能力最强同时对ACE蛋白的影响最大. 本研究说明四条茶叶多肽是通过改变ACE蛋白结构来达到抑制作用, 符合变构抑制剂的作用原理.
Similar content being viewed by others
References
Asoodeh A, Yazdi MM, Chamani J, 2012. Purification and characterisation of angiotensin I converting enzyme inhibitory peptides from lysozyme hydrolysates. Food Chem, 131(1):291–295. https://doi.org/10.1016/j.foodchem.2011.08.039
Bougatef A, Nedjar-Arroume N, Ravallec-Plé R, et al., 2008. Angiotensin I-converting enzyme (ACE) inhibitory activities of sardinelle (Sardinella aurita) by-products protein hydrolysates obtained by treatment with microbial and visceral fish serine proteases. Food Chem, 111(2):350–356. https://doi.org/10.1016/j.foodchem.2008.03.074
Case DA, Cheatham TE III, Darden T, et al., 2005. The amber biomolecular simulation programs. J Computat Chem, 26(16):1668–1688. https://doi.org/10.1002/jcc.20290
Chamata Y, Watson KA, Jauregi P, 2020. Whey-derived peptides interactions with ACE by molecular docking as a potential predictive tool of natural ACE inhibitors. Int J Mol Sci, 21(3):864. https://doi.org/10.3390/ijms21030864
Cheung BMY, Li C, 2012. Diabetes and hypertension: is there a common metabolic pathway? Curr Atheroscler Rep, 14(2):160–166. https://doi.org/10.1007/s11883-012-0227-2
Collier SR, Landram MJ, 2012. Treatment of prehypertension: lifestyle and/or medication. Vasc Health Risk Manag, 8:613–619. https://doi.org/10.2147/vhrm.S29138
Dang YL, Zhou TY, Hao L, et al., 2019. In vitro and in vivo studies on the angiotensin-converting enzyme inhibitory activity peptides isolated from broccoli protein hydrolysate. J Agric Food Chem, 67(24):6757–6764. https://doi.org/10.1021/acs.jafc.9b01137
Daskaya-Dikmen C, Yucetepe A, Karbancioglu-Guler F, et al., 2017. Angiotensin-I-converting enzyme (ACE)-inhibitory peptides from plants. Nutrients, 9(4):316. https://doi.org/10.3390/nu9040316
Ding L, Zhang Y, Jiang YQ, et al., 2014. Transport of egg white ACE-inhibitory peptide, Gln-Ile-Gly-Leu-Phe, in human intestinal Caco-2 cell monolayers with cytoprotective effect. J Agric Food Chem, 62(14):3177–3182. https://doi.org/10.1021/jf405639w
Ding L, Wang LY, Zhang T, et al., 2018. Hydrolysis and transepithelial transport of two corn gluten derived bioactive peptides in human Caco-2 cell monolayers. Food Res Int, 106:475–480. https://doi.org/10.1016/j.foodres.2017.12.080
Escudero E, Mora L, Fraser PD, et al., 2013. Purification and identification of antihypertensive peptides in Spanish dry-cured ham. J Proteomics, 78:499–507. https://doi.org/10.1016/j.jprot.2012.10.019
Ettehad D, Emdin CA, Kiran A, et al., 2016. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet, 387(10022):957–967. https://doi.org/10.1016/s0140-6736(15)01225-8
Fan HB, Wang JP, Liao W, et al., 2019. Identification and characterization of gastrointestinal-resistant angiotensin-converting enzyme inhibitory peptides from egg white proteins. J Agric Food Chem, 67(25):7147–7156. https://doi.org/10.1021/acs.jafc.9b01071
Ferreira SH, 1965. A bradykinin-potentiating factor (BPF) present in the venom of Bothrops jararaca. Br J Pharmacol Chemother, 24(1): 163–169. https://doi.org/10.1111/j.1476-5381.1965.tb02091.x
Forghani B, Zarei M, Ebrahimpour A, et al., 2016. Purification and characterization of angiotensin converting enzyme-inhibitory peptides derived from Stichopus horrens stability study against the ACE and inhibition kinetics. J Funct Foods, 20:276–290. https://doi.org/10.1016/j.jff.2015.10.025
García-Mora P, Martín-Martínez M, Angeles Bonache M, et al., 2017. Identification, functional gastrointestinal stability and molecular docking studies of lentil peptides with dual antioxidant and angiotensin I converting enzyme inhibitory activities. Food Chem, 221:464–472. https://doi.org/10.1016/j.foodchem.2016.10.087
Groneberg DA, Döring F, Eynott PR, et al., 2001. Intestinal peptide transport: ex vivo uptake studies and localization of peptide carrier PEPT1. Am J Physiol-Gastrointestin Liver Physiol, 281(3):G697–G704. https://doi.org/10.1152/ajpgi.2001.281.3.G697
Guan SS, Han WW, Zhang H, et al., 2016. Insight into the interactive residues between two domains of human somatic Angiotensin-converting enzyme and Angiotensin II by MM-PBSA calculation and steered molecular dynamics simulation. J Biomol Struct Dyn, 34(1):15–28. https://doi.org/10.1080/07391102.2015.1007167
Gu Y, Majumder K, Wu JP, 2011. QSAR-aided in silico approach in evaluation of food proteins as precursors of ACE inhibitory peptides. Food Res Int, 44(8):2465–2474. https://doi.org/10.1016/j.foodres.2011.01.051
Gunalan S, Somarathinam K, Bhattacharya J, et al., 2020. Understanding the dual mechanism of bioactive peptides targeting the enzymes involved in renin angiotensin system (RAS): an in-silico approach. J Biomol Struct Dyn, 38(7):5044–5061. https://doi.org/10.1080/07391102.2019.1695668
Hanif K, Bid HK, Konwar R, 2010. Reinventing the ACE inhibitors: some old and new implications of ACE inhibition. Hypertens Res, 33(1): 11–21. https://doi.org/10.1038/hr.2009.184
Harrison C, Acharya KR, 2014. ACE for all—a molecular perspective. J Cell Commun Signal, 8(3):195–210. https://doi.org/10.1007/s12079-014-0236-8
Homeyer N, Gohlke H, 2012. Free energy calculations by the molecular mechanics poisson-boltzmann surface area method. Mol Inform, 31(2):114–122. https://doi.org/10.1002/minf.201100135
Kumar R, Chaudhary K, Chauhan JS, et al., 2015. An in silico platform for predicting, screening and designing of anti-hypertensive peptides. Sci Rep, 5: 12512. https://doi.org/10.1038/srep12512
Lacroix IME, Meng GT, Cheung IWY, et al., 2016. Do whey protein-derived peptides have dual dipeptidyl-peptidase IV and angiotensin I-converting enzyme inhibitory activities? J Funct Foods, 21:87–96. https://doi.org/10.1016/j.jff.2015.11.038
Lafarga T, Hayes M, 2017. Bioactive protein hydrolysates in the functional food ingredient industry: overcoming current challenges. Food Rev Int, 33(3):217–246. https://doi.org/10.1080/87559129.2016.1175013
Laskowski RA, Swindells MB, 2011. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model, 51(10):2778–2786. https://doi.org/10.1021/ci200227u
Li DX, Wang RR, Huang JB, et al., 2019. Effects and mechanisms of tea regulating blood pressure: evidences and promises. Nutrients, 11(5): 1115. https://doi.org/10.3390/nu11051115
Li Y, Sadiq FA, Liu TJ, et al., 2015. Purification and identification of novel peptides with inhibitory effect against angiotensin I-converting enzyme and optimization of process conditions in milk fermented with the yeast Kluyveromyces marxianus. J Funct Foods, 16:278–288. https://doi.org/10.1016/j.jff.2015.04.043
Liu C, Fang L, Min W, et al., 2018. Exploration of the molecular interactions between angiotensin-I-converting enzyme (ACE) and the inhibitory peptides derived from hazelnut (Corylus heterophylla Fisch.). Food Chem, 245:471–480. https://doi.org/10.1016/j.foodchem.2017.10.095
Lu YT, Lu P, Wang Y, et al., 2019. A novel dipeptidyl pepti-dase IV inhibitory tea peptide improves pancreatic β-cell function and reduces α-cell proliferation in streptozotocin-induced diabetic mice. Int J Mol Sci, 20(2):322. https://doi.org/10.3390/ijms20020322
Majumder K, Chakrabarti S, Morton JS, et al., 2015. Egg-derived ACE-inhibitory peptides IQW and LKP reduce blood pressure in spontaneously hypertensive rats. J Funct Foods, 13:50–60. https://doi.org/10.1016/j.jff2014.12.028
Martin M, Deussen A, 2019. Effects of natural peptides from food proteins on angiotensin converting enzyme activity and hypertension. Crit Rev Food Sci Nutr, 59(8):1264–1283. https://doi.org/10.1080/10408398.2017.1402750
Mills KT, Bundy JD, Kelly TN, et al., 2016. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation, 134(6):441–450. https://doi.org/10.1161/circulationaha.115.018912
Miner-Williams WM, Stevens BR, Moughan PJ, 2014. Are intact peptides absorbed from the healthy gut in the adult human? Nutr Res Rev, 27(2):308–329. https://doi.org/10.1017/s0954422414000225
Minkiewicz P, Dziuba J, Iwaniak A, et al., 2008. Biopep database and other programs for processing bioactive peptide sequences. JAOAC Int, 91(4):965–980. https://doi.org/10.1093/jaoac/91.4.965
Ning XY, Zhang YL, Yuan TT, et al., 2018. Enhanced thermostability of glucose oxidase through computer-aided molecular design. Int J Mol Sci, 19(2):425. https://doi.org/10.3390/ijms19020425
Patil P, Mandal S, Tomar SK, et al., 2015. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur J Nutr, 54(6):863–880. https://doi.org/10.1007/s00394-015-0974-2
Puchalska P, Marina Alegre ML, García López MC, 2015. Isolation and characterization of peptides with antihypertensive activity in foodstuffs. Crit Rev Food Sci Nutr, 55(4):521–551. https://doi.org/10.1080/10408398.2012.664829
Regazzo D, Mollé D, Gabai G, et al., 2010. The (193–209) 17-residues peptide of bovine β-casein is transported through Caco-2 monolayer. Mol Nutr Food Res, 54(10): 1428–1435. https://doi.org/10.1002/mnfr.200900443
Saito Y, Wanezaki K, Kawato A, et al., 1994. Structure and activity of angiotensin I converting enzyme inhibitory peptides from sake and sake lees. Biosci Biotechnol Biochem, 58(10):1767–1771. https://doi.org/10.1271/bbb.58.1767
Satoh E, Tohyama N, Nishimura M, 2005. Comparison of the antioxidant activity of roasted tea with green, oolong, and black teas. Int J Food Sci Nutr, 56(8):551–559. https://doi.org/10.1080/09637480500398835
Shalaby SM, Zakora M, Otte J, 2006. Performance of two commonly used angiotensin-converting enzyme inhibition assays using FA-PGG and HHL as substrates. J Dairy Res, 73(2): 178–186. https://doi.org/10.1017/s0022029905001639
Sharma A, Singla D, Rashid M, et al., 2014. Designing of peptides with desired half-life in intestine-like environment. BMC Bioinformatics, 15:282. https://doi.org/10.1186/1471-2105-15-282
Shen YM, Maupetit J, Derreumaux P, et al., 2014. Improved PEP-FOLD approach for peptide and miniprotein structure prediction. J Chem Theory Comput, 10(10):4745–4758. https://doi.org/10.1021/ct500592m
Shimizu M, 1999. Modulation of intestinal functions by food substances. Nahrung, 43(3): 154–158. https://doi.org/10.1002/(SICI)1521-3803(19990601)43:3<154::AID-FOOD154>3.0.CO;2-A
Sornwatana T, Bangphoomi K, Roytrakul S, et al., 2015. Chebulin: Terminalia chebula Retz. fruit-derived peptide with angiotensin-I-converting enzyme inhibitory activity. Biotechnol Appl Biochem, 62(6):746–753. https://doi.org/10.1002/bab.1321
Sun LP, Wu BY, Yan MY, et al., 2019. Antihypertensive effect in vivo of QAGLSPVR and its transepithelial transport through the Caco-2 cell monolayer. Mar Drugs, 17(5):288. https://doi.org/10.3390/md17050288
Tanzadehpanah H, Asoodeh A, Saberi MR, et al., 2013. Identification of a novel angiotensin-I converting enzyme inhibitory peptide from ostrich egg white and studying its interactions with the enzyme. Innovat Food Sci Emerg Technol, 18:212–219. https://doi.org/10.1016/j.ifset.2013.02.002
Tao C, Yu DH, Cornelius V, et al., 2017. Potential health impact and cost-effectiveness of drug therapy for prehypertension. Int J Cardiol, 240:403–408. https://doi.org/10.1016/j.ijcard.2017.05.003
Tao ML, Sun HJ, Liu L, et al., 2017. Graphitized porous carbon for rapid screening of angiotensin-converting enzyme inhibitory peptide GAMVVH from silkworm pupa protein and molecular insight into inhibition mechanism. J Agric Food Chem, 65(39):8626–8633. https://doi.org/10.1021/acs.jafc.7b03195
Trott O, Olson AJ, 2010. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem, 31(2):455–461. https://doi.org/10.1002/jcc.21334
Wang Q, Wang Y, Chen GJ, 2016. Influence of secondary-structure folding on the mutually exclusive folding process of GL5/I27 protein: evidence from molecular dynamics simulations. Int J Mol Sci, 17(11):1962. https://doi.org/10.3390/ijms17111962
Wang YF, Yang ZW, Wei XL, 2010. Sugar compositions, α-glucosidase inhibitory and amylase inhibitory activities of polysaccharides from leaves and flowers of Camellia sinensis obtained by different extraction methods. Int J Biol Macromol, 47(4):534–539. https://doi.org/10.1016/j.ijbiomac.2010.07.007
Wu QY, Jia JQ, Yan H, et al., 2015. A novel angiotensin-I converting enzyme (ACE) inhibitory peptide from gastrointestinal protease hydrolysate of silkworm pupa (Bombyx mori) protein: biochemical characterization and molecular docking study. Peptides, 68:17–24. https://doi.org/10.1016/j.peptides.2014.07.026
Yu DY, Wang C, Song YF, et al., 2019. Discovery of novel angiotensin-converting enzyme inhibitory peptides from Todarodes pacificus and their inhibitory mechanism: in silico and in vitro studies. Int J Mol Sci, 20(17):4159. https://doi.org/10.3390/ijms20174159
Zhang ZF, Li Q, Liang J, et al., 2010. Epigallocatechin-3-O-gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition. Phytomedicine, 17(1): 14–18. https://doi.org/10.1016/j.phymed.2009.09.007
Zhao TR, Liu BT, Yuan L, et al., 2019. ACE inhibitory activity in vitro and antihypertensive effect in vivo of LSGYGP and its transepithelial transport by Caco-2 cell monolayer. J Funct Foods, 61:103488. https://doi.org/10.1016/j.jff.2019.103488
Zheng YJ, Wang X, Zhuang YL, et al., 2019. Isolation of novel ACE-inhibitory and antioxidant peptides from quinoa bran albumin assisted with an in silico approach: characterization, in vivo antihypertension, and molecular docking. Molecules, 24(24):4562. https://doi.org/10.3390/molecules24244562
Zheng YJ, Wang X, Zhuang YL, et al., 2020. Isolation of novel ACE-inhibitory peptide from naked oat globulin hydrolysates in silico approach: molecular docking, in vivo antihypertension and effects on renin and intracellular endothelin-1. J Food Sci, 85(4): 1328–1337. https://doi.org/10.1111/1750-3841.15115
Acknowledgments
The research was supported by the National Key Research and Development Program of China (No. 2016YFD0200900) and the Science Technology Department of Zhejiang Province (No. 2016C02053-8), China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Yating LU performed the experimental research and data analysis, and wrote and edited the manuscript. Yu WANG, Danyi HUANG, and Dongmei FAN performed the data analysis. Zhuang BIAN performed the experimental research. Peng LU reviewed and edited the manuscript. Xiaochang WANG supervised the research, and reviewed and edited the manuscript. All authors have read and approved the final manuscript and, therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Yating LU, Yu WANG, Danyi HUANG, Zhuang BIAN, Peng LU, Dongmei FAN, and Xiaochang WANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Lu, Y., Wang, Y., Huang, D. et al. Inhibitory mechanism of angiotensin-converting enzyme inhibitory peptides from black tea. J. Zhejiang Univ. Sci. B 22, 575–589 (2021). https://doi.org/10.1631/jzus.B2000520
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B2000520
Key words
- Black tea
- Angiotensin-1-converting enzyme (ACE) inhibitory peptide
- Kinetic study
- Molecular docking
- Molecular dynamic (MD) simulation