Abstract
Immunomagnetic bead (IMB)-based enzyme-linked immunosorbent assay (ELISA) has been the tool frequently used for protein detection in research and clinical laboratories. For most ELISA reactions the recommended dosage of IMBs is usually according to their weight (mg) or mass fraction (w/v) instead of the bead number. Consequently, the processes occurring in the immediate vicinity of the IMBs have always been ignored by researchers and they cannot be revealed in detail during the ELISA reaction. In this paper, we established the relationship between number of IMBs and colorimetric results, and further proposed a new concept of “nominal effective immunoreaction volume (NEIV)” to characterize a single IMB during ELISA reaction. Results showed that the NEIV of a single IMB has a constant value, which is unrelated to the amount of beads and the concentration of antigen. Optimal results of the colorimetric ELISA are achieved when the incubation volume meets each IMB’s NEIV and is no longer enhanced by increasing the incubation volume. Thus, the reliable and relatively precise number of IMBs for ELISA detection during practical application could be determined. Most importantly, a study using IMB’s NEIV would lay the foundation for a kinetics analysis of IMBs and antigens for future study.
摘要
目 的
通过探究单个磁珠的有效免疫反应体积 (NEIV), 在微观水平上揭示磁珠的免疫反应过程, 为大体积样本检测过程中磁珠的用量提供理论指导。
创新点
首次提出 “单个磁珠的有效免疫反应体积” 这一概念, 提出在微观水平上对磁珠免疫反应进行探究, 为进一步研究磁珠-抗原免疫反应的动力学过程打下基础。
方 法
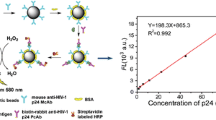
首先, 采用两种直径的磁珠对50 μl 不同浓度的抗原进行比色检测, 证明磁珠上抗原的结合位点过量。 随后, 采用定量磁珠对相同浓度不同体积的抗原进行检测, 得出“单个磁珠的有效免疫反应体积”这一概念。 接着, 采用三种不同用量的磁珠对浓度相同、体积梯度增大的抗原进行检测, 以验证所提出的NEIV 的概念。 然后, 通过测定孵育时长、孵育温度和震荡与否对比色结果的影响, 找到对NEIV 影响的关键因素。 最后采用定量磁珠, 对梯度稀释的抗原进行检测, 建立磁珠用量与比色结果的关系, 对NEIV 的实际应用提出展望。
结 论
对于同一直径的磁珠, 在相同的孵育条件下, 其单个磁珠的NEIV 是恒定的, 与磁珠用量和孵育体积无关。 孵育温度、孵育时长和震荡与否是影响NEIV 值的关键因素。 采用NEIV 这一参数可以在大体积样本检测之前, 对磁珠的用量模拟提供有效指导。 除此之外, NEIV 更从微观角度上揭示了磁珠和抗原的免疫反应过程, 为进一步研究磁珠-抗原免疫反应的动力学过程奠定了基础。
Similar content being viewed by others
References
Chen, Y.P., Xianyu, Y.L., Wang, Y., et al., 2015. One-step detection of pathogens and viruses: combining magnetic relaxation switching and magnetic separation. ACS Nano, 9(3): 3184–3191. http://dx.doi.org/10.1021/acsnano.5b00240
Cohen, N., Sabhachandani, P., Golberg, A., et al., 2015. Approaching near real-time biosensing: microfluidic microsphere based biosensor for real-time analyte detection. Biosens. Bioelectron., 66: 454–460. http://dx.doi.org/10.1016/j.bios.2014.11.018
Kim, M.H., Choi, S.J., 2015. Immunoassay of paralytic shellfish toxins by moving magnetic particles in a stationary liquid-phase lab-on-a-chip. Biosens. Bioelectron., 66: 136–140. https://doi.org/10.1016/j.bios.2014.11.012
Kourilov, V., Steinitz, M., 2002. Magnetic-bead enzyme-linked immunosorbent assay verifies adsorption of ligand and epitope accessibility. Anal. Biochem., 311(2): 166–170. http://dx.doi.org/10.1016/S0003-2697(02)00405-0
Li, D.Y., Ying, Y.B., Wu, J., et al., 2013. Comparison of monomeric and polymeric horseradish peroxidase as labels in competitive ELISA for small molecule detection. Microchim. Acta, 180(7–8): 711–717. http://dx.doi.org/10.1007/s00604-013-0974-y
Makaraviciute, A., Ramanaviciene, A., 2013. Site-directed antibody immobilization techniques for immunosensors. Biosens. Bioelectron., 50: 460–471. http://dx.doi.org/10.1016/j.bios.2013.06.060
Monaci, L., Brohée, M., Tregoat, V., et al., 2011. Influence of baking time and matrix effects on the detection of milk allergens in cookie model food system by ELISA. Food Chem., 127(2): 669–675. http://dx.doi.org/10.1016/j.foodchem.2010.12.113
Nishi, H., Nishimura, S., Higashiura, M., et al., 2000. A new method for histamine release from purified peripheral blood basophils using monoclonal antibody-coated magnetic beads. J. Immunol. Methods, 240(1–2): 39–46. http://dx.doi.org/10.1016/S0022-1759(00)00169-1
Parsa, H., Chin, C.D., Mongkolwisetwara, P., et al., 2008. Effect of volume-and time-based constraints on capture of analytes in microfluidic heterogeneous immunoassays. Lab Chip, 8(12): 2062–2070. http://dx.doi.org/10.1039/b813350f
Peng, J.M., Su, Y.L., Chen, W.J., et al., 2013. Polyamide nanofiltration membrane with high separation performance prepared by EDC/NHS mediated interfacial polymerization. J. Membr. Sci., 427(1): 92–100. http://dx.doi.org/10.1016/j.memsci.2012.09.039
Pieper, J.S., Hafmans, T., Veerkamp, J.H., et al., 2000. Development of tailor-made collagen-glycosaminoglycan matrices: EDC/NHS crosslinking, and ultrastructural aspects. Biomaterials, 21(6): 581–593. http://dx.doi.org/10.1016/S0142-9612(99)00222-7
Saha, B., Evers, T.H., Prins, M.W., 2014. How antibody surface coverage on nanoparticles determines the activity and kinetics of antigen capturing for biosensing. Anal. Chem., 86(16): 8158–8166. http://dx.doi.org/10.1021/ac501536z
Shih, C.H., Wu, H.C., Chang, C.Y., et al., 2014. An enzymelinked immunosorbent assay on a centrifugal platform using magnetic beads. Biomicrofluidics, 8(5): 052110. http://dx.doi.org/10.1063/1.4896297
Song, F., Zhou, Y., Li, Y.S., et al., 2014. A rapid immunomagnetic beads-based immunoassay for the detection of β-casein in bovine milk. Food Chem., 158: 445–448. http://dx.doi.org/10.1016/j.foodchem.2014.02.150
Tran, Q.H., Nguyen, T.H., Mai, A.T., et al., 2012. Development of electrochemical immunosensors based on different serum antibody immobilization methods for detection of Japanese encephalitis virus. Adv. Nat. Sci.: Nanosci. Nanotechnol., 3(1): 015012.
Urusov, A.E., Petrakova, A.V., Vozniak, M.V., et al., 2014. Rapid immunoenzyme assay of aflatoxin B1 using magnetic nanoparticles. Sensors, 14(11): 21843–21857. http://dx.doi.org/10.3390/s141121843
Wang, S.Q., Tasoglu, S., Chen, P.Z., et al., 2014. Micro-afluidics ELISA for rapid CD4 cell count at the pointof-care. Sci. Rep., 4: 3796. http://dx.doi.org/10.1038/srep03796
Wang, T.Y., Zhang, M.H., Dreher, D.D., et al., 2013. Ultrasensitive microfluidic solid-phase ELISA using an actuatable microwell-patterned PDMS chip. Lab Chip, 13(21): 4190–4197. http://dx.doi.org/10.1039/c3lc50783a
Xiong, Q., Cui, X., Saini, J.K., et al., 2014. Development of an immunomagnetic separation method for efficient enrichment of Escherichia coli O157:H7. Food Control, 37: 41–45. http://dx.doi.org/10.1016/j.foodcont.2013.08.033
Xu, J., Yin, W.W., Zhang, Y.Y., et al., 2012. Establishment of magnetic beads-based enzyme immunoassay for detection of chloramphenicol in milk. Food Chem., 134(4): 2526–2531. http://dx.doi.org/10.1016/j.foodchem.2012.04.083
Yang, W., Kernstock, R., Simmons, N., et al., 2015. ELISA microplate: a viable immunocapture platform over magnetic beads for immunoaffinity-LC-MS/MS quantitation of protein therapeutics. Bioanalysis, 7(3): 307–318. http://dx.doi.org/10.4155/bio.14.250
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Natural Science Foundation of China (No. 31571918), Hong Kong, Macao, and Taiwan Scientific and Technological Cooperation Projects (No. 2015DFT30150) of China, and the Zhejiang Provincial Department of Education (No. Y201533676), China
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, R., Chen, Y., Fan, K. et al. Nominal effective immunoreaction volume of magnetic beads at single bead level. J. Zhejiang Univ. Sci. B 18, 845–853 (2017). https://doi.org/10.1631/jzus.B1600358
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1600358
Key words
- Nominal effective immunoreaction volume (NEIV)
- Immunomagnetic bead
- Enzyme-linked immunosorbent assay (ELISA)
- Bead diameter
- Mixing mode