Abstract
Objective
Bovine endometritis is one of the most common reproductive disorders in cattle. The aim of this study was to investigate the anti-inflammation potential of punicalagin in lipopolysaccharide (LPS)-induced bovine endometrial epithelial cells (bEECs) and to uncover the underlying mechanisms.
Methods
bEECs were stimulated with different concentrations (1, 10, 30, 50, and 100 μg/ml) of LPS for 3, 6, 9, 12, and 18 h. MTT assay was used to assess cell viability and to identify the conditions for inflammatory injury and effective concentrations of punicalagin. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to assess gene expression of pro-inflammatory cytokines. Western blotting was used to assess levels of inflammation-related proteins.
Results
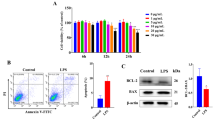
Treatment of bEECs with 30 μg/ml LPS for 12 h induced cell injury and reduced cell viability. Punicalagin (5, 10, or 20 μg/ml) pretreatment significantly decreased LPS-induced productions of interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α (TNF-α) in bEECs. Molecular research showed that punicalagin inhibited the activation of the upstream mediator nuclear factor-κB (NF-κB) by suppressing the production of inhibitor κBα (IκBα) and phosphorylation of p65. Results also indicated that punicalagin can suppress the phosphorylation of mitogen-activated protein kinases (MAPKs) including p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK).
Conclusions
Punicalagin may attenuate LPS-induced inflammatory injury and provide a potential option for the treatment of dairy cows with Escherichia coli endometritis.
摘要
目的
评估安石榴苷对脂多糖诱导奶牛子宫内膜上皮细 胞炎症损伤的保护作用,并初步探讨其作用机 制。
创新点
首次证明安石榴苷对脂多糖诱导奶牛子宫内膜上 皮细胞炎症损伤具有保护作用,且此作用与核转 录因子κB(NF-κB)和丝裂原活化蛋白激酶 (MAPK)信号通路的抑制相关。
方法
用不同浓度的脂多糖(1、10、30、50 和100 μg/ml) 刺激奶牛子宫内膜上皮细胞3、6、9、12 和18 h, 筛选出建立炎症损伤的最佳作用浓度和时间。 安石榴苷预处理细胞2 h 后用脂多糖刺激12 h, 用逆转录聚合酶链式反应(RT-PCR)检测炎症 因子白细胞介素1β(IL-1β)、白细胞介素6(IL-6)、 白细胞介素8(IL-8)及肿瘤坏死因子α(TNF-α) 的表达。用蛋白质免疫印迹试验(Western blotting) 的方法检测核因子κB 抑制蛋白α(IκBα)、 磷酸化的p65、p38、c-Jun 氨基末端激酶(JNK) 和细胞外调节蛋白激酶(ERK)的表达水平。
结论
MTT 结果显示,30 μg/ml 脂多糖刺激奶牛子宫内 膜上皮细胞12 h能够造成细胞活力下降和形态改 变(图2 和3)。RT-PCR 结果显示,安石榴苷预 处理后炎症因子显著降低(图4)。Western blotting 结果显示,安石榴苷预处理能显著抑制IκBα 降 解以及p65、p38、JNK 和ERK 的磷酸化表达水 平(图5 和6)。综上所述,安石榴苷对脂多糖 诱导奶牛子宫内膜上皮细胞炎症损伤具有保护 作用,在治疗奶牛子宫内膜炎中具有重要价值。
Similar content being viewed by others
References
Aqil, F., Munagala, R., Vadhanam, M.V., et al., 2012. Antiproliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res. Int., 49(1):345–353. http://dx.doi.org/10.1016/j.foodres.2012.07.059
Azawi, O.I., 2008. Postpartum uterine infection in cattle. Anim. Reprod. Sci., 105(3-4):187–208. http://dx.doi.org/10.1016/j.anireprosci.2008.01.010
Brodzki, P., Bochniarz, M., Brodzki, A., et al., 2014. Trueperella pyogenes and Escherichia coli as an etiological factor of endometritis in cows and the susceptibility of these bacteria to selected antibiotics. Pol. J. Vet. Sci., 17(4):657–664.
Chapwanya, A., Meade, K.G., Doherty, M.L., et al., 2013. Endometrial epithelial cells are potent producers of tracheal antimicrobial peptide and serum amyloid A3 gene expression in response to E. coli stimulation. Vet. Immunol. Immunopathol., 151(1-2):157–162. http://dx.doi.org/10.1016/j.vetimm.2012.09.042
Checker, R., Patwardhan, R.S., Sharma, D., et al., 2012. Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-κB. Free Radic. Biol. Med., 53(7): 1421–1430. http://dx.doi.org/10.1016/j.freeradbiomed.2012.08.006
Choi, H.J., Kang, O.H., Park, P.S., et al., 2007. Mume fructus water extract inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated macrophages. J. Med. Food, 10(3):460–466. http://dx.doi.org/10.1089/jmf.2006.198
Corbetta, S., Vicentini, L., Ferrero, S., et al., 2005. Activity and function of the nuclear factor kappaB pathway in human parathyroid tumors. Endocr. Relat. Cancer, 12(4): 929–937. http://dx.doi.org/10.1677/erc.1.00970
Fu, Y., Liu, B., Feng, X., et al., 2013. Lipopolysaccharide increases Toll-like receptor 4 and downstream Toll-like receptor signaling molecules expression in bovine endometrial epithelial cells. Vet. Immunol. Immunopathol., 151(1-2):20–27. http://dx.doi.org/10.1016/j.vetimm.2012.09.039
Gu, J.H., Ge, J.B., Li, M., et al., 2012. Inhibition of NF-κB activation is associated with anti-inflammatory and antiapoptotic effects of Ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. Eur. J. Pharm. Sci., 47(4):652–660. http://dx.doi.org/10.1016/j.ejps.2012.07.016
Herath, S., Fischer, D.P., Werling, D., et al., 2006. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology, 147(1):562–570. http://dx.doi.org/10.1210/en.2005-1113
Hines, D.J., Choi, H.B., Hines, R.M., et al., 2013. Prevention of LPS-induced microglia activation, cytokine production and sickness behavior with TLR4 receptor interfering peptides. PLoS ONE, 8(3):e60388. http://dx.doi.org/10.1371/journal.pone.0060388
Huang, B., Xiao, D., Tan, B., et al., 2016. Chitosan oligosaccharide reduces intestinal inflammation that involves calcium-sensing receptor (CaSR) activation in lipopolysaccharide (LPS)-challenged piglets. J. Agric. Food Chem., 64(1):245–252. http://dx.doi.org/10.1021/acs.jafc.5b05195
Janowski, T., Baranski, W., Lukasik, K., et al., 2013. Prevalence of subclinical endometritis in repeat breeding cows and mRNA expression of tumor necrosis factor a and inducible nitric oxide synthase in the endometrium of repeat breeding cows with and without subclinical endometritis. Pol. J. Vet. Sci., 16(4):693–699. http://dx.doi.org/10.2478/pjvs-2013-0098
Jean-Gilles, D., Li, L., Vaidyanathan, V.G., et al., 2013. Inhibitory effects of polyphenol punicalagin on type-II collagen degradation in vitro and inflammation in vivo. Chem. Biol. Interact., 205(2):90–99. http://dx.doi.org/10.1016/j.cbi.2013.06.018
Kasimanickam, R.K., Kasimanickam, V.R., Olsen, J.R., et al., 2013. Associations among serum pro-and anti-inflammatory cytokines, metabolic mediators, body condition, and uterine disease in postpartum dairy cows. Reprod. Biol. Endocrinol., 11(1):103. http://dx.doi.org/10.1186/1477-7827-11-103
Kawai, T., Akira, S., 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity, 34(5):637–650. http://dx.doi.org/10.1016/j.immuni.2011.05.006
Khan, K.N., Kitajima, M., Hiraki, K., et al., 2009. Toll-like receptors in innate immunity: role of bacterial endotoxin and Toll-like receptor 4 in endometrium and endometriosis. Gynecol. Obstet. Invest., 68(1):40–52. http://dx.doi.org/10.1159/000212061
Kulkarni, A.P., Mahal, H.S., Kapoor, S., et al., 2007. In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. J. Agric. Food Chem., 55(4):1491–1500. http://dx.doi.org/10.1021/jf0626720
Kumar, H., Kaur, A., Kishor, N., et al., 2016. Prevalence of multiple antibiotic resistant nasal carriage MRSA among healthy population of border villages in Amritsar Region, Punjab, India. J. Clin. Diagn. Res., 10(5):DL01–DL02. http://dx.doi.org/10.7860/JCDR/2016/16131.7781
Lim, J.Y., Hwang, B.Y., Hwang, K.W., et al., 2012. Methylalpinumisoflavone inhibits lipopolysaccharide-induced inflammation in microglial cells by the NF-kappaB and MAPK signaling pathway. Phytother. Res., 26(12):1948–1956. http://dx.doi.org/10.1002/ptr.4810
Ling, M., Li, Y., Xu, Y., et al., 2012. Regulation of miRNA-21 by reactive oxygen species-activated ERK/NF-κB in arsenite-induced cell transformation. Free Radic. Biol. Med., 52(9):1508–1518. http://dx.doi.org/10.1016/j.freeradbiomed.2012.02.020
Liu, M., Song, S., Li, H., et al., 2014. The protective effect of caffeic acid against inflammation injury of primary bovine mammary epithelial cells induced by lipopolysaccharide. J. Dairy Sci., 97(5):2856–2865. http://dx.doi.org/10.3168/jds.2013-7600
Mackeen, A.D., Packard, R.E., Ota, E., et al., 2015. Antibiotic regimens for postpartum endometritis. Cochrane Database Syst. Rev., (2):CD001067. http://dx.doi.org/10.1002/14651858.CD001067.pub3
Malinowski, E., Lassa, H., Markiewicz, H., et al., 2011. Sensitivity to antibiotics of Arcanobacterium pyogenes and Escherichia coli from the uteri of cows with metritis/endometritis. Vet. J., 187(2):234–238. http://dx.doi.org/10.1016/j.tvjl.2009.12.010
Morimoto, Y., Kikuchi, K., Ito, T., et al., 2009. MK615 attenuates Porphyromonas gingivalis lipopolysaccharideinduced pro-inflammatory cytokine release via MAPK inactivation in murine macrophage-like RAW264.7 cells. Biochem. Biophys. Res. Commun., 389(1):90–94. http://dx.doi.org/10.1016/j.bbrc.2009.08.103
Olajide, O.A., Kumar, A., Velagapudi, R., et al., 2014. Punicalagin inhibits neuroinflammation in LPS-activated rat primary microglia. Mol. Nutr. Food Res., 58(9):1843–1851. http://dx.doi.org/10.1002/mnfr.201400163
Pandrea, I., Xu, C., Stock, J.L., et al., 2016. Antibiotic and antiinflammatory therapy transiently reduces inflammation and hypercoagulation in acutely SIV-infected pigtailed macaques. PLoS Pathog., 12(1):e1005384. http://dx.doi.org/10.1371/journal.ppat.1005384
Peng, J., Wei, D., Fu, Z., et al., 2015. Punicalagin ameliorates lipopolysaccharide-induced acute respiratory distress syndrome in mice. Inflammation, 38(2):493–499. http://dx.doi.org/10.1007/s10753-014-9955-5
Qi, Z., Qi, S., Ling, L., et al., 2016. Salidroside attenuates inflammatory response via suppressing JAK2-STAT3 pathway activation and preventing STAT3 transfer into nucleus. Int. Immunopharmacol., 35:265–271. http://dx.doi.org/10.1016/j.intimp.2016.04.004
Risco, A., del Fresno, C., Mambol, A., et al., 2012. p38 and p38d kinases regulate the Toll-like receptor 4 (TLR4)-induced cytokine production by controlling ERK1/2 protein kinase pathway activation. Proc. Natl. Acad. Sci. USA, 109(28):11200–11205. http://dx.doi.org/10.1073/pnas.1207290109
Ruimi, N., Rwashdeh, H., Wasser, S., et al., 2010. Daedalea gibbosa substances inhibit LPS-induced expression of iNOS by suppression of NF-κB and MAPK activities in RAW 264.7 macrophage cells. Int. J. Mol. Med., 25(3): 421–432.
Sens, A., Heuwieser, W., 2013. Presence of Escherichia coli, Trueperella pyogenes, α-hemolytic streptococci, and coagulase-negative staphylococci and prevalence of subclinical endometritis. J. Dairy Sci., 96(10):6347–6354. http://dx.doi.org/10.3168/jds.2013-6646
Sheldon, I.M., Roberts, M.H., 2010. Toll-like receptor 4 mediates the response of epithelial and stromal cells to lipopolysaccharide in the endometrium. PLoS ONE, 5(9): e12906. http://dx.doi.org/10.1371/journal.pone.0012906
Sheldon, I.M., Lewis, G.S., LeBlanc, S., et al., 2006. Defining postpartum uterine disease in cattle. Theriogenology, 65(8):1516–1530. http://dx.doi.org/10.1016/j.theriogenology.2005.08.021
Shimada, K., Daida, H., Ma-Krupa, W., et al., 2005. Lipopolysaccharide, CD14 and Toll-like receptors: an emerging link between innate immunity and atherosclerotic disease. Future Cardiol., 1(5):657–674. http://dx.doi.org/10.2217/14796678.1.5.657
Soboll, G., Schaefer, T.M., Wira, C.R., 2006. Effect of Toll-like receptor (TLR) agonists on TLR and microbicide expression in uterine and vaginal tissues of the mouse. Am. J. Reprod. Immunol., 55(6):434–446. http://dx.doi.org/10.1111/j.1600-0897.2006.00381.x
Taguri, T., Tanaka, T., Kouno, I., 2004. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull., 27(12):1965–1969. http://dx.doi.org/10.1248/bpb.27.1965
Turner, M.L., Cronin, J.G., Healey, G.D., et al., 2014. Epithelial and stromal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via Toll-like receptors TLR2, TLR1, and TLR6. Endocrinology, 155(4):1453–1465. http://dx.doi.org/10.1210/en.2013-1822
Ugurlu, O., Yaris, M., Oztekin, C.V., et al., 2010. Impacts of antibiotic and anti-inflammatory therapies on serum prostate-specific antigen levels in the presence of prostatic inflammation: a prospective randomized controlled trial. Urol. Int., 84(2):185–190. http://dx.doi.org/10.1159/000277596
Wagener, K., Grunert, T., Prunner, I., et al., 2014. Dynamics of uterine infections with Escherichia coli, Streptococcus uberis and Trueperella pyogenes in post-partum dairy cows and their association with clinical endometritis. Vet. J., 202(3):527–532. http://dx.doi.org/10.1016/j.tvjl.2014.08.023
Wang, H.W., Wu, T., Qi, J.Y., et al., 2013. Salidroside attenuates LPS-stimulated activation of THP-1 cell-derived macrophages through down-regulation of MAPK/NF-κB signaling pathways. J. Huazhong Univ. Sci. Technol. Med. Sci., 33(4):463–469. http://dx.doi.org/10.1007/s11596-013-1143-6
Wang, Z., Jiang, W., Zhang, Z., et al., 2012. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J. Ethnopharmacol., 144(1):145–150. http://dx.doi.org/10.1016/j.jep.2012.08.041
Ward, E., Duff, P., 2016. A comparison of 3 antibiotic regimens for prevention of postcesarean endometritis: an historical cohort study. Am. J. Obstet. Gynecol., 214(6): 751.e1-751.e4. http://dx.doi.org/10.1016/j.ajog.2016.02.037
Wira, C.R., Grant-Tschudy, K.S., Crane-Godreau, M.A., 2005. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am. J. Reprod. Immunol., 53(2):65–76. http://dx.doi.org/10.1111/j.1600-0897.2004.00248.x
Wu, H., Zhao, G., Jiang, K., et al., 2016. Engeletin alleviates lipopolysaccharide-induced endometritis in mice by inhibiting TLR4-mediated NF-κB activation. J. Agric. Food Chem., 64(31):6171–6178. http://dx.doi.org/10.1021/acs.jafc.6b02304
Xu, L., He, S., Yin, P., et al., 2016. Punicalagin induces Nrf2 translocation and HO-1 expression via PI3K/Akt, protecting rat intestinal epithelial cells from oxidative stress. Int. J. Hyperthermia, 32(5):465–473. http://dx.doi.org/10.3109/02656736.2016.1155762
Xu, X., Yin, P., Wan, C., et al., 2014. Punicalagin inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4-mediated MAPKs and NF-κB activation. Inflammation, 37(3):956–965. http://dx.doi.org/10.1007/s10753-014-9816-2
Yaidikar, L., Thakur, S., 2015. Punicalagin attenuated cerebral ischemia-reperfusion insult via inhibition of proinflammatory cytokines, up-regulation of Bcl-2, down-regulation of Bax, and caspase-3. Mol. Cell. Biochem., 402(1-2): 141–148. http://dx.doi.org/10.1007/s11010-014-2321-y
Yaidikar, L., Byna, B., Thakur, S.R., 2014. Neuroprotective effect of punicalagin against cerebral ischemia reperfusioninduced oxidative brain injury in rats. J. Stroke Cerebrovasc. Dis., 23(10):2869–2878. http://dx.doi.org/10.1016/j.jstrokecerebrovasdis.2014.07.020
Yang, Y., Xiu, J., Zhang, L., et al., 2012. Antiviral activity of punicalagin toward human enterovirus 71 in vitro and in vivo. Phytomedicine, 20(1):67–70. http://dx.doi.org/10.1016/j.phymed.2012.08.012
Yoon, Y.K., Park, D.W., Sohn, J.W., et al., 2016. Effects of inappropriate empirical antibiotic therapy on mortality in patients with healthcare-associated methicillin-resistant Staphylococcus aureus bacteremia: a propensity-matched analysis. BMC Infect. Dis., 16(1):331. http://dx.doi.org/10.1186/s12879-016-1650-8
Zhao, H.X., Zhao, J.L., Shen, J.Z., et al., 2014. Prevalence and molecular characterization of fluoroquinolone resistance in Escherichia coli isolates from dairy cattle with endometritis in China. Microb. Drug. Resist., 20(2): 162–169. http://dx.doi.org/10.1089/mdr.2013.0073
Acknowledgments
We are thankful for help from the members of China Agricultural University-Beijing University of Agriculture Traditional Chinese Veterinary Medicine (CAU-BUA TCVM) teaching and research team.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Project supported by the National Key Technology R & D Program of China (No. 2013BAD10B04) and the Importation and Development of High-Caliber Talents Project of Beijing Municipal Institutions (No. CIT&TCD20130324), China
ORCID: An LYU, http://orcid.org/0000-0002-3268-5193
Rights and permissions
About this article
Cite this article
Lyu, A., Chen, Jj., Wang, Hc. et al. Punicalagin protects bovine endometrial epithelial cells against lipopolysaccharide-induced inflammatory injury. J. Zhejiang Univ. Sci. B 18, 481–491 (2017). https://doi.org/10.1631/jzus.B1600224
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1600224