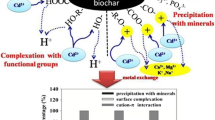
Abstract
The study on biochar derived from plant biomass for environmental applications is attracting more and more attention. Twelve sets of biochar were obtained by treating four phytoremediation plants, Salix rosthornii Seemen, Thalia dealbata, Vetiveria zizanioides, and Phragmites sp., sequentially through pyrolysis at 500 °C in a N2 environment, and under different temperatures (500, 600, and 700 °C) in a CO2 environment. The cation exchange capacity and specific surface area of biochar varied with both plant species and pyrolysis temperature. The magnesium (Mg) content of biochar derived from T. dealbata (TC) was obviously higher than that of the other plant biochars. This biochar also had the highest sorption capacity for phosphate and ammonium. In terms of biomass yields, adsorption capacity, and energy cost, T. dealbata biochar produced at 600 °C (TC600) is the most promising sorbent for removing contaminants (N and P) from aqueous solution. Therefore, T. dealbata appears to be the best candidate for phytoremediation application as its biomass can make a good biochar for environmental cleaning.
Similar content being viewed by others
References
Abe, K., Ozaki, Y., 1998. Comparison of useful terrestrial and aquatic plant species for removal of nitrogen and phosphorus from domestic wastewater. Soil Sci. Plant Nutr., 44(4):599–607. [doi:10.1080/00380768.1998.1041 4483]
Arias, C., Del Bubba, M., Brix, H., 2001. Phosphorus removal by sands for use as media in subsurface flow constructed reed beds. Water Res., 35(5):1159–1168. [doi:10.1016/S0043-1354(00)00368-7]
Brix, H., 1997. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol., 35(5):11–18. [doi:10.1016/S0273-1223(97)00047-4]
Cao, X., Harris, W., 2010. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresource Technol., 101(14):5222–5228. [doi:10.1016/j.biortech.2010.02.052]
Chun, Y., Sheng, G., Chiou, C.T., Xing, B., 2004. Compositions and sorptive properties of crop residue-derived chars. Environ. Sci. Technol., 38(17):4649–4655. [doi:10.1021/es035034w]
Conley, D.J., Paerl, H.W., Howarth, R.W., Boesch, D.F., Seitzinger, S.P., Havens, K.E., Lancelot, C., Likens, G.E., 2009. Controlling eutrophication: nitrogen and phosphorus. Science, 323(5917):1014–1015. [doi:10.1126/science.1167755]
Demirbas, A., 2004. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrol., 72(2):243–248. [doi:10.1016/j.jaap.2004.07.003]
Ding, Y., Liu, Y.X., Wu, W.X., Shi, D.Z., Yang, M., Zhong, Z.K., 2010. Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water Air Soil Pollut., 213(1–4):47–55. [doi:10.1007/s11270-010-0366-4]
Eberhardt, T.L., Min, S.H., Han, J.S., 2006. Phosphate removal by refined aspen wood fiber treated with carboxymethyl cellulose and ferrous chloride. Bioresource Technol., 97(18):2371–2376. [doi:10.1016/j.biortech.2005.10.040]
Gerritse, R.G., 1993. Prediction of travel times of phosphate in soils at a disposal site for wastewater. Water Res., 27(2): 263–267. [doi:10.1016/0043-1354(93)90084-U]
Glaser, B., Lehmann, J., Zech, W., 2002. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal-a review. Biol. Fert. Soils, 35(4):219–230. [doi:10.1007/s00374-002-0466-4]
Hossain, M.K., Strezov, V., Chan, K.Y., Ziolkowski, A., Nelson, P.F., 2011. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manage., 92(1):223–228. [doi:10.1016/j.jenvman.2010.09.008]
Inyang, M., Gao, B., Yao, Y., Xue, Y., Zimmerman, A.R., Pullammanappallil, P., Cao, X., 2012. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresource Technol., 110:50–56. [doi:10.1016/j.biortech.2012.01.072]
Kameyama, K., Miyamoto, T., Shiono, T., Shinogi, Y., 2011. Influence of sugarcane bagasse-derived biochar application on nitrate leaching in calcaric dark red soil. J. Environ. Qual., 41(4):1131–1137. [doi:10.2134/jeq2010.0453]
Karaosmanoglu, F., Işigigür-Ergüdenler, A., Sever, A., 2000. Biochar from the straw-stalk of rapeseed plant. Energy Fuels, 14(2):336–339. [doi:10.1021/ef9901138]
Kumar, S., Loganathan, V.A., Gupta, R.B., Barnett, M.O., 2011. An assessment of U(VI) removal from groundwater using biochar produced from hydrothermal carbonization. J. Environ. Manage., 92(10):2504–2512. [doi:10.1016/j.jenvman.2011.05.013]
Lehmann, J., da Silva, J.P.Jr., Steiner, C., Nehls, T., Zech, W., Glaser, B., 2003. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil, 249(2):343–357. [doi:10.1023/A: 1022833116184]
Liang, B., Lehmann, J., Solomon, D., Kinyangi, J., Grossman, J., O’Neill, B., Skjemstad, J.O., Thies, J., Luizão, F.J., Petersen, J., 2006. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J., 70(5): 1719–1730. [doi:10.2136/sssaj2005.0383]
Lu, Q., He, Z.L., Graetz, D.A., Stoffella, P.J., Yang, X., 2010. Phytoremediation to remove nutrients and improve eutrophic stormwaters using water lettuce (Pistia stratiotes L.). Environ. Sci. Pollut. Res., 17(1):84–96. [doi:10.1007/s11356-008-0094-0]
Mohan, D., Pittman, C.U., Bricka, M., Smith, F., Yancey, B., Mohammad, J., Steele, P.H., Alexandre-Franco, M.F., Gómez-Serrano, V., Gong, H., 2007. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Coll. Interf. Sci., 310(1):57–73. [doi:10.1016/j.jcis.2007.01.020]
Pignatello, J.J., Kwon, S., Lu, Y., 2006. Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): attenuation of surface activity by humic and fulvic acids. Environ. Sci. Technol., 40(24):7757–7763. [doi:10.1021/es061307m]
Pulido-Novicio, L., Hata, T., Kurimoto, Y., Doi, S., Ishihara, S., Imamura, Y., 2001. Adsorption capacities and related characteristics of wood charcoals carbonized using a one-step or two-step process. J. Wood Sci., 47(1):48–57. [doi:10.1007/BF00776645]
Ravikovitch, P.I., Neimark, A.V., 2001. Characterization of nanoporous materials from adsorption and desorption isotherms. Coll. Surface A, 187–188:11–21. [doi:10.1016/S0927-7757(01)00614-8]
Schollenberger, C., Simon, R., 1945. Determination of exchange capacity and exchangeable bases in soil-ammonium acetate method. Soil Sci., 59(1):13–24.
Seo, B.S., Park, C.M., Song, U., Park, W.J., 2010. Nitrate and phosphate removal potentials of three willow species and a bald cypress from eutrophic aquatic environment. Landscape Ecol. Eng., 6(2):211–217. [doi:10.1007/s11355-009-0102-7]
Uchimiya, M., Lima, I.M., Thomas Klasson, K., Chang, S.C., Wartelle, L.H., Rodgers, J.E., 2010. Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J. Agric. Food Chem., 58(9):5538–5544. [doi:10.1021/jf9044217]
Valipour, A., Kalyan Raman, V., Ghole, V.S., 2009. A new approach in wetland systems for domestic wastewater treatment using Phragmites sp. Ecol. Eng., 35(12): 1797–1803. [doi:10.1016/j.ecoleng.2009.08.004]
Valix, M., Cheung, W., Mckay, G., 2004. Preparation of activated carbon using low temperature carbonisation and physical activation of high ash raw bagasse for acid dye adsorption. Chemosphere, 56(5):493–501. [doi:10.1016/j.chemosphere.2004.04.004]
Walton, K.S., Snurr, R.Q., 2007. Applicability of the BET method for determining surface areas of microporous metal-organic frameworks. J. Am. Chem. Soc., 129(27): 8552–8556. [doi:10.1021/ja071174k]
Wilkie, A.C., Evans, J.M., 2010. Aquatic plants: an opportunity feedstock in the age of bioenergy. Biofuels, 1(2): 311–321. [doi:10.4155/bfs.10.2]
Xu, X., Cao, X., Zhao, L., Wang, H., Yu, H., Gao, B., 2013. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res., 20(1):358–368. [doi:10.1007/s11356-012-0873-5]
Yang, X., Wu, X., Hao, H., He, Z., 2008. Mechanisms and assessment of water eutrophication. J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 9(3):197–209. [doi:10.1631/jzus.B0710626]
Yao, Y., Gao, B., Inyang, M., Zimmerman, A.R., Cao, X., Pullammanappallil, P., Yang, L., 2011a. Biochar derived from anaerobically digested sugar beet tailings: characterization and phosphate removal potential. Bioresource Technol., 102(10):6273–6278. [doi:10.1016/j.biortech.2011.03.006]
Yao, Y., Gao, B., Inyang, M., Zimmerman, A.R., Cao, X., Pullammanappallil, P., Yang, L., 2011b. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater., 190(1–3):501–507. [doi:10.1016/j.jhazmat.2011.03.083]
Yuan, J.H., Xu, R.K., Zhang, H., 2011. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresource Technol., 102(3):3488–3497. [doi:10.1016/j.biortech.2010.11.018]
Zhao, F., Yang, W., Zeng, Z., Li, H., Yang, X., He, Z., Gu, B., Rafiq, M.T., Peng, H., 2012. Nutrient removal efficiency and biomass production of different bioenergy plants in hypereutrophic water. Biomass Bioenergy, 42:212–218. [doi:10.1016/j.biombioe.2012.04.003]
Author information
Authors and Affiliations
Corresponding authors
Additional information
The two authors contributed equally to this work
Project supported by the International Cooperative Project from the Ministry of Science and Technology of China (No. 2010DFB33960), the National Key Technology R&D Program of China (No. 2012BAC17B02), the Zhejiang Youth Creative Program (No. 2012QNA6004), and the Key Project from Zhejiang Science and Technology Bureau (No. 2011C13015), China
Rights and permissions
About this article
Cite this article
Zeng, Z., Zhang, Sd., Li, Tq. et al. Sorption of ammonium and phosphate from aqueous solution by biochar derived from phytoremediation plants. J. Zhejiang Univ. Sci. B 14, 1152–1161 (2013). https://doi.org/10.1631/jzus.B1300102
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1300102