Abstract
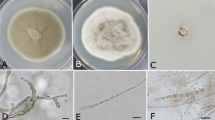
Endophytic flora plays a vital role in the colonization and survival of host plants, especially in harsh environments, such as arid regions. This flora may, however, contain pathogenic species responsible for various troublesome host diseases. The present study is aimed at investigating the diversity of both cultivable and non-cultivable endophytic fungal floras in the internal tissues (roots and leaves) of Tunisian date palm trees (Phoenix dactylifera). Accordingly, 13 isolates from both root and leaf samples, exhibiting distinct colony morphology, were selected from potato dextrose agar (PDA) medium and identified by a sequence match search wherein their 18S–28S internal transcribed spacer (ITS) sequences were compared to those available in public databases. These findings revealed that the cultivable root and leaf isolates fell into two groups, namely Nectriaceae and Pleosporaceae. Additionally, total DNA from palm roots and leaves was further extracted and ITS fragments were amplified. Restriction fragment length polymorphism (RFLP) analysis of the ITS from 200 fungal clones (leaves: 100; roots: 100) using HaeIII restriction enzyme revealed 13 distinct patterns that were further sequenced and led to the identification of Alternaria, Cladosporium, Davidiella (Cladosporium teleomorph), Pythium, Curvularia, and uncharacterized fungal endophytes. Both approaches confirmed that while the roots were predominantly colonized by Fusaria (members of the Nectriaceae family), the leaves were essentially colonized by Alternaria (members of the Pleosporaceae family). Overall, the findings of the present study constitute, to the authors’ knowledge, the first extensive report on the diversity of endophytic fungal flora associated with date palm trees (P. dactylifera).
Similar content being viewed by others
References
Abdelghani, E.Y., Bala, K., Paul, B., 2004. Characterization of Pythium paroecandrum and its antagonism towards Botrytis cinerea, the causative agent of grey mould disease of grape. FEMS Microbiol. Lett., 230(2):177–183. [doi:10.1016/S0378-1097(03)00895-4]
Alabouvette, C., Olivain, C., Migheli, Q., Steinberg, C., 2009. Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol., 184(3):529–544. [doi:10.1111/j.1469-8137.2009.03014.x]
Altschul, S.F., Gish, W., Miller, W., Myers, E.W., Lipman, D.J., 1990. Basic local alignment search tool. J. Mol. Biol., 215(3):403–410. [doi:10.1016/S0022-2836(05)80360-2]
Ampe, F., Ben Omar, N., Moizan, C., Wacher, C., Guyot, J.P., 1999. Polyphasic study of the special distribution of microorganisms in Mexican pozol, a fermented maize dough, demonstrates the need for cultivation-independent methods to investigate traditional fermentations. Appl. Environ. Microbiol., 65(12):5464–5473.
Arenal, F., Platas, G., Pelaez, F., 2005. Preussia africana and Preussia pseudominima, two new Preussia species defined based on morphological and molecular evidences. Fungal Divers., 20:1–15.
Arenal, F., Platas, G., Peláez, F., 2007. A new endophytic species of Preussia (Sporormiaceae) inferred from morphological observations and molecular phylogenetic analysis. Fungal Divers., 25:1–17.
Arnold, A.E., Maynard, Z., Gilbert, G.S., Coley, P.D., Kursar, T.A., 2000. Are tropical fungal endophytes hyperdiverse? Ecol. Lett., 3:267–274.
Arx, J.A., 1949. Beitrage zur kenntnis der gattung Mycosphaerella. Sydowia, 3:28–100 (in German).
Backman, P.A., Sikora, R.A., 2008. Endophytes: an emerging tool for biological control. Biol. Control, 46(1):1–3. [doi:10.1016/j.biocontrol.2008.03.009]
Bacon, C.W., Porter, J.K., Robbins, J.D., Luttrell, E.S., 1977. Epichloe typhina from toxic tall fescue grasses. Appl. Environ. Microbiol., 34:576–581.
Bacon, C.W., Porter, J.K., Robbins, J.D., 1979. Laboratory production of ergot alkaloids by species of Balansia. J. Gen. Microbiol., 113(1):119–126. [doi:10.1099/0022 1287-113-1-119]
Bensch, K., Groenewald, J.Z., Dijksterhuis, J., Starink-Willemse, M., Andersen, B., Summerell, B.A., Shin, H.D., Dugan, F.M., Schroers, H.J., Braun, U., et al., 2010. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Stud. Mycol., 67(1):1–94. [doi:10.3114/sim.2010.67.01]
Bimboim, H.C., Doly, J., 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucl. Acids Res., 7(6):1513–1523. [doi:10.1093/nar/7.6.1513]
Braun, R.A., Crous, P.W., Dugan, F., Groenewald, J.Z., de Hoog, G.S., 2003. Phylogeny and taxonomy of Cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s. str. Mycol. Prog., 2(1): 3–18. [doi:10.1007/s11557-006-0039-2]
Cain, R.F., 1961. Studies of coprophilous ascomycetes. VII. Preussia. Can. J. Bot., 39(7):1633–1666. [doi:10.1139/b61-144]
Camara, M.P.S., Palm, M.E., van Berkum, P., O’Neill, N.R., 2002. Molecular phylogeny of Leptosphaeria and Phaeosphaeria. Mycologia, 94(4):630–640. [doi:10.2307/3761714]
Cao, L.X., You, J.L., Zhou, S.N., 2002. Endophytic fungi from Musa acuminata leaves and roots in South China. World J. Microbiol. Biotechnol., 18(2):169–171. [doi:10.1023/A:1014491528811]
Collins, C.H., Lyne, P.M., 1984. Microbiological Methods, 5th Ed. Butterworths Co., Ltd., London, p.56–113.
Coppola, S., Blaiotta, G., Ercolini, D., Moschetti, G., 2001. Molecular evaluation of microbial diversity occurring in different types of Mozzarella cheese. J. Appl. Microbiol., 90(3):414–420. [doi:10.1046/j.1365-2672.2001.01262.x]
Crous, P.W., Braun, U., Schubert, K., Groenewald, J.Z., 2007. Delimiting Cladosporium from morphologically similar genera. Stud. Mycol., 58(1):33–56. [doi:10.3114/sim.2007.58.02]
Crous, P.W., Braun, U., Wingfield, M.J., Wood, A.R., Shin, H.D., 2009. Phylogeny and taxonomy of obscure genera of microfungi. Persoonia, 22:139–161.
de Cock, A.W., Mendoza, L., Padhye, A.A., Ajello, L., Kaufman, L., 1987. Pythium insidiosum sp. nov., the etiologic agent of pythiosis. J. Clin. Microbiol., 25(2): 344–349.
Dellaporta, S.L., Wood, J., Hicks, J.B., 1983. A plant DNA minipreparation: version II. Plant Mol. Biol. Rep., 1(4): 19–21. [doi:10.1007/BF02712670]
Dickie, I.A., FitzJohn, R.G., 2007. Using terminal restriction fragment length polymorphism (T-RFLP) to identify mycorrhizal fungi: a methods review. Mycorrhiza, 17(4): 259–270. [doi:10.1007/s00572-007-0129-2]
Dimuthu, S.M., Lei, C., Eric, H.C.M., Pedro, W.C., Hugo, M., Ekachai, C., Roger, G.S., Yu, P.T., Kevin, D.H., 2012. A phylogenetic and taxonomic re-evaluation of the Bipolaris-Cochliobolus-Curvularia complex. Fungal Divers., 56(1):131–144. [doi:10.1007/s13225-012-0189-2]
Dini-Andreote, F., Pietrobon, V.C., Dini Andreote, F., Romão, A.S., Spósito, M.B., Araújo, W.L., 2009. Genetic variability of Brazilian isolates of Alternaria alternata detected by AFLP and RAPD techniques. Braz. J. Microbiol., 40(3):670–677. [doi:10.1590/S1517-83822009000300032]
Dixon, L.J., Schlub, R.L., Pernezny, K., Datnoff, L.E., 2009. Host specialization and phylogenetic diversity of Corynespora cassiicola. Phytopathology, 99(9):1015–1027. [doi:10.1094/PHYTO-99-9-1015]
Drira, N., Benbadis, A., 1985. Vegetative multiplication of date-palm (Phoenix dactylifera L.) by reversion of in vitro cultured female flower buds. J. Plant. Physiol., 119(3): 227–235. [doi:10.1016/S0176-1617(85)80182-6]
Dwivedi, S.P., Husain, N., Singh, R.B., Mall, N., 2012. 18S ribosomal DNA based PCR diagnostic assay for Trichomonas vaginalis infection in symptomatic and asymptomatic women in India. Asian Pac. J. Trop. Dis., 2(2):133–138. [doi:10.1016/S2222-1808(12)60031-0]
El Hassni, M., El Hadrami, A., Daayf, F., Chérif, M., Barka, E.A., El Hadrami, I., 2007. Biological control of Bayoud disease in date palm: selection of microorganisms inhibiting the causal agent and inducing defense reactions. Environ. Exp. Bot., 59(2):224–234. [doi:10.1016/j.envexpbot.2005.12.008]
Felske, A., Wolterink, A., van Lis, R., de Vos, W.M., Akkermans, A.D.L., 1999. Searching for predominant soil bacteria: 16S rDNA cloning versus strain cultivation. FEMS Microbiol. Ecol., 30(2):137–145. [doi:10.1111/j.1574-6941.1999.tb00642.x]
Fernandes, M.R.V., Costae Silva, T.A., Pfenning, L.H., da Costa-Neto, C.M., Heinrich, T.A., de Alencar, S.M., de Lima, M.A., Ikegaki, M., 2009. Biological activities of the fermentation extract of the endophytic fungus Alternaria alternate isolated from Coffea Arabica L. Braz. J. Pharm. Sci., 45(4):677–685. [doi:10.1590/S1984-82502009000400010]
Fernandez, D., Lourd, M., Ouinten, M., Tantaoui, A., Geiger, J.P., 1995. Le Bayoud du palmier dattier: une maladie qui menace la phoéniciculture. Phytoma, 469:36–39 (in French).
Feurer, C., Vallaeys, T., Corrieu, G., Irlinger, F., 2004. Does smearing inoculum reflect the bacterial composition of the smear at the end of the ripening of a French soft, red-smear cheese? J. Dairy Sci., 87(10):3189–3197. [doi:10.3168/jds.S0022-0302(04)73454-2]
Fisher, P.J., Petrini, O., Petrini, L.E., Descals, E., 1992. A preliminary study of fungi inhabiting xylem and whole stems of Olea europaea. Sydowia, 44:117–221.
Fisher, P.J., Petrini, L.E., Sutton, B.C., Petrini, O., 1995. A study of fungal endophytes in leaves, stems and roots of Gynoxis oleifolia Muchler (Compositae) from Ecuador. Nova Hedwigia, 60:589–594.
Gao, X.X., Zhou, H., Xu, D.Y., Yu, C.H., Chen, Y.Q., Qu, L.H., 2005. High diversity of endophytic fungi from the pharmaceutical plant, Heterosmilax japonica Kunth revealed by cultivation-independent approach. FEMS Microbiol. Lett., 249(2):255–266. [doi:10.1016/j.femsle.2005.06.017]
Gherbawy, Y.A.M.H., Adler, A., Prillinger, H., 2002. Genotypic identification of Fusarium subglutinans, F. proliferatum and F. verticillioides strains isolated from maize in Austria. Mycobiology, 30(3):139–145. [doi:10.4489/MYCO.2002.30.3.139]
Girlanda, M., Ghignone, S., Luppi, A.M., 2002. Diversity of sterile root-associated fungi of two Mediterranean plants. New Phytol., 155(3):481–498. [doi:10.1046/j.1469-8137.2002.00474.x]
Gond, S.K., Verma, V.C., Kumar, A., Kumar, V., Kharwar, R.N., 2007. Study of endophytic fungal community from different parts of Aegle marmelos Correae (Rutaceae) from Varanasi (India). World J. Microbiol. Biotechnol., 23(10):1371–1375. [doi:10.1007/s11274-007-9375-x]
Groger, D., 1972. Ergot. In: Kadis, S., Ciegler, A., Ajl, S.J. (Eds.), Microbial Toxins. Academic Press, New York, Vol. 8, p.321–373.
Guo, L.D., Hyde, K.D., Liew, E.C.Y., 2001. Detection and taxonomic placement of endophytic fungi within frond tissues of Livistona chinensis based on rDNA sequences. Mol. Phylogenet. Evol., 20(1):1–13. [doi:10.1006/mpev.2001.0942]
Hatimi, A., 1989. Etude de la Réceptivité des Sols de Deux Palmeraies Marocaines au Bayoud. PhD Thesis, Cadi Ayyad University, Marrakech, p.58 (in French).
Hibar, K., Edel-Herman, V., Steinberg, C., Gautheron, N., Daami-Remadi, M., Alabouvette, C., El Mahjoub, M., 2007. Genetic diversity of Fusarium oxysporum populations isolated from tomato plants in Tunisia. J. Phytopathol., 155(3):136–142. [doi:10.1111/j.1439-0434.2007.01198.x]
Huang, W.Y., Cai, Y.Z., Surveswaran, S., Hyde, K.D., Corke, H., Sun, M., 2009. Molecular phylogenetic identification of endophytic fungi isolated from three Artemisia species. Fungal Divers., 36:69–88.
Hugenholtz, P., Goebel, B.M., Pace, N.R., 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol., 180:4765–4774.
Jones, E.E., Deacon, J.W., 1995. Comparative physiology and behaviour of the mycoparasites Pythium acanthophoron, P. oligandrum and P. mycoparasiticum. Biocontr. Sci. Technol., 5(1):27–39. [doi:10.1080/09583159550039990]
Khan, R., Shahzad, S., Choudhary, M.I., Khan, S.A., Ahmad, A., 2007. Biodiversity of the endophytic fungi isolated from Calotropis procera (Ait.) R. Br. Pak. J. Bot., 39(6):2233–2239.
Kodsueb, R., Dhanasekaran, V., Aptroot, A., Lumyong, S., McKenzie, E.H.C., Hyde, K.D., Jeewon, R., 2006. The family Pleosporaceae: intergeneric relationships and phylogenetic perspectives based on sequence analyses of partial 28S rDNA. Mycologia, 98(4):571–583. [doi:10.3852/mycologia.98.4.571]
Kwasna, H., Ward, E., Kosiak, B., 2006. Lewia hordeicola sp. nov. from barley grain. Mycologia, 98(4):662–668. [doi:10.3852/mycologia.98.4.662]
Lamberti, F., 1988. Etiology of Two Diseases of the Date Palm at Tozeur and Nefta. Report to the Government of Tunisia.
Larran, S., Monaco, C., Alippi, H.E., 2001. Endophytic fungi in leaves of Lycopersicon esculentum Mill. World J. Microbiol. Biotechnol., 17(2):181–184. [doi:10.1023/A:1016670000288]
Larran, S., Perello, A., Simon, M.R., Moreno, V., 2002a. Isolation and analysis of endophytic microorganisms in wheat (Triticum aestivum L.) leaves. World J. Microbiol. Biotechnol., 18(7):683–686. [doi:10.1023/A:1016857917950]
Larran, S., Rollán, C., Bruno Angeles, H., Alippi, H.E., Urrutia, M.I., 2002b. Nota Corta: endophytic fungi in healthy soybean leaves. Invest. Agr.: Prod. Prot. Veg., 17(1): 173–177.
Lee, K.S., Portman, J.L., Sweeney, C.A., Cheung, A.T.W., 2009. Fungal endophytes: an untapped natural products source of cytotoxic agents. FASEB J., 23 (Meeting Abstract Supplement):711.5.
Lévesque, C.A., de Cock, A.W., 2004. Molecular phylogeny and taxonomy of the genus Pythium. Mycol. Res., 108(12):1363–1383. [doi:10.1017/S0953756204001431]
Lim, P.O., Woo, H.R., Nam, H.G., 2003. Molecular genetics of leaf senescence in Arabidopsis. Trends Plant Sci., 8(6): 272–278. [doi:10.1016/S1360-1385(03)00103-1]
Lucero, M.E., Unc, A., Cooke, P., Dowd, S., Sun, S., 2011. Endophyte microbiome diversity in micropropagated Atriplex canescens and Atriplex torreyi var. griffithsii. PLoS ONE, 6(3):e17693. [doi:10.1371/journal.pone.0017693]
Manamgoda, D.S., Cai, L., McKenzie, E.H.C., Crous, P.W., Madrid, H., Chukeatirote, E., Shivas, G., Tan, Y.P., Hyde, K.D., 2012. A phylogenetic and taxonomic re-evaluation of the Bipolaris-Cochliobolus-Curvularia complex. Fungal Divers., 56(1):131–144. [doi:10.1007/s13225-012-0189-2]
Namsi, A., Montarone, M., Serra, P., Ben Mahamoud, O., Takrouni, M.L., Zouba, A., Khoualdia, O., Bové, J.M., Duran-Vila, N., 2007. Manganese and brittle leaf disease of palm date trees. J. Plant Pathol., 89(1):125–136.
Paul, B., 2004. A new species of Pythium isolated from Burgundian vineyards and its antagonism towards Botrytis cinerea, the causative agent of the grey mould disease. FEMS Microbiol. Lett., 234(2):269–274. [doi:10.1111/j.1574-6968.2004.tb09543.x]
Peláez, F., Collado, J., Arenal, F., Basilio, A., Cabello, A., Díez, M.T., García, J.B., González del Val, A., González, V., Gorrochategui, J., et al., 1998. Endophytic fungi from plants living on gypsum soils as a source of secondary metabolites with antimicrobial activity. Mycol. Res., 102(6):755–761. [doi:10.1017/S0953756297005662]
Petrini, O., 1991. Fungal Endophytes of Tree Leaves. In: Andrews, J., Hirano, S. (Eds.), Microbial Ecology of Leaves. Springer, Berlin Heidelberg, New York, p.179–197. [doi:10.1007/978-1-4612-3168-4_9]
Pocasangre, L., Sikora, R.A., Vilich, V., Schuster, R.P., 2000. Survey of banana endophytic fungi from Central America and screening for biological control of the burrowing nematode (Radopholus similis). Acta Hort., 53:283–290.
Porras-Alfaro, A., Herrera, J., Sinsabaugh, R.L., Odenbach, K.J., Lowrey, T., Natvig, D.O., 2008. Novel root fungal consortium associated with a dominant desert grass. Appl. Environ. Microbiol., 74(9):2805–2813. [doi:10.1128/AEM.02769-07]
Pryor, B.M., Bigelow, D.M., 2003. Molecular characterization of Embellisia and Nimbya species and their relationship to Alternaria, Ulocladium, and Stemphylium. Mycologia, 95(6):1141–1154. [doi:10.2307/3761916]
Rana, B.K., Singh, U.P., Taneja, D., 1997. Antifungal activity and kinetics of inhibition by essential oil isolated from leaves of Aegle marmelos. J. Ethnopharmacol., 57(1): 29–34. [doi:10.1016/S0378-8741(97)00044-5]
Rodrigues, A., Mueller, U.G., Ishak, H.D., Bacci, M.Jr., Pagnocca, F.C., 2011. Ecology of microfungal communities in gardens of fungus-growing ants (Hymenoptera: Formicidae): a year-long survey of three species of attine ants in Central Texas. FEMS Microbiol. Ecol., 78(2):244–255. [doi:10.1111/j.1574-6941.2011.01152.x]
Rodrigues, A., Passarini, M.R.Z., Ferro, M., Nagamoto, N.S., Forti, L.C., Bacci, M.Jr., Sette, L.D., Pagnocca, F.C., 2013. Fungal communities in the garden chamber soils of leaf-cutting ants. J. Basic Microbiol., online. [doi:10. 1002/jobm.201200458]
Rosa, L.H., Vaz, A.B.M., Caligiorne, R.B., Campolina, S., Rosa, C.A., 2009. Endophytic fungi associated with the Antarctic grass Deschampsia antarctica Desv. (Poaceae). Polar Biol., 32(2):161–167. [doi:10.1007/s00300-008-0515-z]
Rungjindamai, N., Pinruan, U., Choeyklin, R., Hattori, T., Jones, E.B.G., 2008. Molecular characterization of basidiomycetous endophytes isolated from leaves, rachis and petioles of the oil palm, Elaeis guineensis, in Thailand. Fungal Divers., 33:139–161.
Saunders, G.A., Washburn, J.O., Egerter, D.E., Anderson, J.A., 1988. Pathogenicity of fungi isolated from field collected larvae of the western tree hole mosquito, Aedes sierrensis (Diptera: Culicidae). J. Invertebr. Pathol., 52(2):360–363. [doi:10.1016/0022-2011(88)90148-6]
Schroeder, H.W., Cole, R.J., 1977. Natural occurrence of alternariols in discolored pecans. J. Agric. Food Chem., 25(1):204–206. [doi:10.1021/jf60209a032]
Schroers, H.J., O’Donnell, K., Lamprecht, S.C., Kammeyer, P.L., Johnson, S., Sutton, D.A., Rinaldi, M.G., Geiser, D.M., Summerbell, R.C., 2009. TTaxonomy and phylogeny of the Fusarium dimerum species group. Mycologia, 101(1):44–70. [doi:10.3852/08-002]
Schulz, B., Wanke, U., Draeger, S., Aust, H.J., 1993. Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycol. Res., 97(12): 1447–1450. [doi:10.1016/S0953-7562(09)80215-3]
Shweta, S., Zuehlke, S., Ramesha, B.T., Priti, V., Kumar, P.M., Ravikanth, G., Spiteller, M., Vasudeva, R., Shaanker, R.U., 2010. Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry, 71(1):117–122. [doi:10.1016/j.phytochem.2009.09.030]
Sigler, W.V., Zeyer, J., 2002. Microbial diversity and activity along the fore fields of two receding glaciers. Microbial Ecol., 43(4):397–407. [doi:10.1007/s00248-001-0045-5]
Smit, E., Leeflang, P., Glandorf, B., van Elsas, J.D., Wernars, K., 1999. Analysis of fungal diversity in the wheat rhizosphere by sequencing of cloned PCR-amplified genes encoding 18S rRNA and temperature gradient gel electrophoresis. Appl. Environ. Microbiol., 65(6): 2614–2621.
Sonjak, S., Beguiristain, T., Leyval, C., Regvar, M., 2009. Temporal temperature gradient gel electrophoresis (TTGE) analysis of arbuscular mycorrhizal fungi associated with selected plants from saline and metal polluted environments. Plant Soil, 314(1–2):25–34. [doi:10.1007/s11104-008-9702-5]
Steinberg, C., Edel, V., Gautheron, N., Vallaeys, T., Alabouvette, C., 1997a. Characterization of Fusarium oxysporum Populations by Growth Parameters Evaluation in Microtiter Plates. In: Dehne, H.W., Adam, G., Diekmann, M., Frahm, J., Mauler-Machnik, A. (Eds.), Diagnosis and Identification of Plant Pathogens. Kluwer Academic Publisher, Dordrecht, p.535–538. [doi:10.1007/978-94-009-0043-1_119]
Steinberg, C., Edel, V., Gautheron, N., Abadie, C., Vallaeys, T., Alabouvette, C., 1997b. Phenotypic characterization of natural populations of Fusarium oxysporum in relation to genotypic characterization. FEMS Microbiol. Ecol., 24(1):73–85. [doi:10.1111/j.1574-6941.1997.tb00424.x]
Strobel, G., Daisy, B., 2003. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev., 67(4):491–502. [doi:10.1128/MMBR.67.4.491-502.2003]
Sutthisa, W., Sanoamuang, N., Chuprayoon, S., 2010. Evaluation of amplified rDNA restriction analysis (ARDRA) for the identification of Fusarium species, the causal agent associated with mulberry root rot disease in Northeastern Thailand. J. Agric. Technol., 6(2):379–390.
Tambong, J.T., de Cock, A.W.A.M., Tinker, N.A., Levesque, C.A., 2006. Oligonucleotide array for identification and detection of Pythium species. Appl. Environ. Microbiol., 72(4):2691–2706. [doi:10.1128/AEM.02183-06]
Tamura, K., Dudley, J., Nei, M., Kumar, S., 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol., 24(8):1596–1599. [doi:10.1093/molbev/msm092]
Tao, G., Liu, Z.Y., Hyde, K.D., Lui, X.Z., Yu, Z.N., 2008. Whole rDNA analysis reveals novel and endophytic fungi in Bletilla ochracea (Orchidaceae). Fungal Divers., 33:101–122.
Taylor, J.E., Hyde, K.D., Jones, E.B.G., 1999. Endophytic fungi associated with the temperate palm, Trachycarpus fortunei, within and outside its natural geographic range. New Phytol., 142(2):335–346. [doi:10.1046/j.1469-8137.1999.00391.x]
Thomma, B.P.H.J., 2003. Alternaria spp.: from general saprophyte to species parasite. Mol. Plant Pathol., 4(4): 225–236. [doi:10.1046/j.1364-3703.2003.00173.x]
Thompson, J.D., Higgins, D.G., Gibson, T.J., 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucl. Acids Res., 22(22):4673–4680. [doi:10.1093/nar/22.22.4673]
Vallaeys, T., Topp, E., Muyzer, G., Macheret, V., Laguerre, G., Rigaud, A., Soulas, G., 1997. Evaluation of denaturing gradient gel electrophoresis in the detection of 16S rDNA sequence variation in rhizobia and methanotrophs. FEMS Microbiol. Ecol., 24(3):279–285. [doi:10.1111/j.1574-6941.1997.tb00445.x]
Wang, J.M., Yang, J.M., Zhu, J.H., Jia, Q.J., Tao, Y.Z., 2010. Assessment of genetic diversity by simple sequence repeat markers among forty elite varieties in the germplasm for malting barley breeding. J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 11(10):792–800. [doi:10.1631/jzus.B0900414]
Wang, Y., Zeng, Q.G., Zhang, Z.B., Yan, R.M., Wang, L.Y., Zhu, D., 2011. Isolation and characterization of endophytic huperzine A-producing fungi from Huperzia serrata. J. Ind. Microbiol. Biotechnol., 38(9):1267–1278. [doi:10.1007/s10295-010-0905-4]
Watanabe, M., Yonezawa, T., Lee, K.I., Kumagai, S., Sugita-Konishi, Y., Goto, K., Hara-Kud, Y., 2011. Molecular phylogeny of the higher and lower taxonomy of the Fusarium genus and differences in the evolutionary histories of multiple genes. BMC Evol. Biol., 11(1):322. [doi:10.1186/1471-2148-11-322]
Webber, J., 1981. A natural biological control of Dutch elm disease. Nature, 292(5822):449–451. [doi:10.1038/292 449a0]
White, T.J., Bruns, T., Lee, S., Taylor, J., 1990. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J. (Eds.), PCR Protocols: A Guide to Methods and Applications. Academic Press Inc., New York, p.315–322.
Wilson, D., 1995. Endophyte: the evolution of a term and clarification of its use and definition. Oikos, 73(2): 274–276. [doi:10.2307/3545919]
Yan, X.N., Sikora, R.A., Zheng, J.W., 2011. Potential use of cucumber (Cucumis sativus L.) endophytic fungi as seed treatment agents against root-knot nematode Meloidogyne incognita. J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 12(3):219–225. [doi:10.1631/jzus.B1000165]
Zhang, F., Li, L., Niu, S., Si, Y., Guo, L., Jiang, X., Che, Y., 2012. A thiopyranchromenone and other chromone derivatives from an endolichenic fungus, Preussia africana. J. Nat. Prod., 75(2):230–237. [doi:10.1021/np2009362]
Zhang, T., Xiang, H.B., Zhang, Y.Q., Liu, H.Y., Wei, Y.Z., Zhao, L.X., Yu, L.Y., 2013. Molecular analysis of fungal diversity associated with three bryophyte species in the Fildes Region, King George Island, maritime Antarctica. Extremophiles, 17(5):757–765. [doi:10.1007/s00792-013-0558-0]
Zhang, Y., Schoch, C.L., Fournier, J., Crous, P.W., de Gruyter, J., Woudenberg, J.H.C., Hirayama, K., Tanaka, K., Pointing, S.B., Spatafora, J.W., et al., 2009a. Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Stud. Mycol., 64(1):85–102. [doi:10.3114/sim.2009.64.04]
Zhang, Y., Wang, H.K., Fournier, J., Crous, P.W., Jeewon, R., Pointing, S.B., Hyde, K.D., 2009b. Towards a phylogenetic clarification of Lophiostoma/Massarina and morphologically similar genera in the Pleosporales. Fungal Divers., 38:225–251.
Zumstein, E., Moletta, R., Godon, J.J., 2000. Examination of two years of community dynamics in an anaerobic bioreactor using fluorescence polymerase chain reaction (PCR) single-strand conformation polymorphism analysis. Environ. Microbiol., 2(1):69–78. [doi:10.1046/j.1462-2920.2000.00072.x]
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by EGIDE (No. 18470SA), CMCU (No. 08G908), and the Tunisian Ministry of Higher Education
Rights and permissions
About this article
Cite this article
Ben Chobba, I., Elleuch, A., Ayadi, I. et al. Fungal diversity in adult date palm (Phoenix dactylifera L.) revealed by culture-dependent and culture-independent approaches. J. Zhejiang Univ. Sci. B 14, 1084–1099 (2013). https://doi.org/10.1631/jzus.B1200300
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1200300
Key words
- Date palm tree (Phoenix dactylifera)
- Endophytic cultivable fungi
- rDNA internal transcribed spacer (ITS)
- Phylogenetic analysis
- Total DNA diversity analysis