Abstract
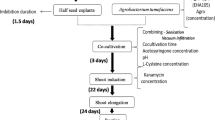
The Agrobacterium-mediated transformation system is the most commonly used method in soybean transformation. Screening of soybean genotypes favorable for Agrobacterium-infection and tissue regeneration is the most important step to establish an efficient genetic transformation system. In this study, twenty soybean genotypes that originated from different soybean production regions in China were screened for transient infection, regeneration capacity, and stable transgenic efficiency. Three genotypes, Yuechun 04-5, Yuechun 03-3, and Tianlong 1, showed comparable stable transgenic efficiencies with that of the previously reported American genotypes Williams 82 and Jack in our experimental system. For the Tianlong 1, the average stable transformation efficiency is 4.59%, higher than that of control genotypes (Jack and Williams 82), which is enough for further genomic research and genetic engineering. While polymerase chain reaction (PCR), LibertyLink strips, and β-glucuronidase (GUS) staining assays were used to detect the insertion and expression of the transgene, leaves painted with 135 mg/L Basta could efficiently identify the transformants.
Similar content being viewed by others
References
Cheng, M., Lowe, B.A., Spencer, T.M., Ye, X.D., Armstrong, C.L., 2004. Factors influencing Agrobacterium-mediated transformation of monocotyledonous species. In Vitro Cell. Dev. Biol.-Plant, 40(1):31–45. [doi:10.1079/IVP2003501]
Dai, S.H., Zheng, P., Marmey, P., Zhang, S.P., Tian, W.Z., Chen, S.Y., Beachy, R.N., Fauquet, C., 2001. Comparative analysis of transgenic rice plants obtained by Agrobacterium-mediated transformation and particle bombardment. Mol. Breeding, 7(1):25–33. [doi:10.1023/A:1009687511633]
Donaldson, P.A., Simmonds, D.H., 2000. Susceptibility to Agrobacterium tumefaciens and cotyledonary node transformation in short-season soybean. Plant Cell Rep., 19(5):478–484. [doi:10.1007/s002990050759]
Doyle, J.J., Doyle, J.L., 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull., 19:11–15.
Edwards, K., Johnstone, C., Thompson, C., 1991. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res., 19(6):1349. [doi:10.1093/nar/19.6.1349]
Finer, J., McMullen, M., 1991. Transformation of soybean via particle bombardment of embryogenic suspension culture tissue. In Vitro Cell. Dev. Biol.-Plant, 27(4):175–182. [doi:10.1007/bf02632213]
Frame, B.R., Shou, H.X., Chikwamba, R.K., Zhang, Z., Xiang, C., Fonger, T.M., Pegg, S.E.K., Li, B., Nettleton, D.S., Pei, D., et al., 2002. Agrobacterium tumefaciens-mediated transformation of maize embryos using standard binary vector system. Plant Physiol., 129(1):13–22. [doi:10.1104/pp.000653]
Gamborg, O.L., Miller, R.A., Ojiama, K., 1968. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res., 50(1):151–158. [doi:10.1016/0014-4827(68)90403-5]
Gelvin, S.B., 2000. Agrobacterium and plant genes involved in T-DNA transfer and integration. Ann. Rev. Plant Physiol. Plant Mol. Biol., 51(1):223–256. [doi:10.1146/annurev.arplant.51.1.223]
Gelvin, S.B., 2003. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev., 67(1):16–37. [doi:10.1128/MMBR.67.1.16-37.2003]
Hinchee, M.A.W., Conner-Ward, D.V., Newell, C.A., 1988. Production of transgenic soybean plants using Agrobacterium-mediated DNA transfer. Nat. Biotechnol., 6(8):915–922. [doi:10.1038/nbt0888-915]
Hood, E.E., Helmer, G.L., Fraley, R.T., Chilton, M.D., 1986. The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J. Bacteriol., 168(3):1291–1301.
James, C., 2012. 2011 ISAAA report on global status of Biotech/GM crops. China Biotechnol., 32(1):1–14 (in Chinese).
Jefferson, R.A., Kavanagh, T.A., Bevan, M.W., 1987. GUS fusion: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J., 6:3901–3907.
Liu, H.C., Wei, Z.M., 2005. Recent advances in soybean genetic transformation. J. Plant Physiol. Mol. Biol., 31(2): 126–134 (in Chinese).
Ma, L., Zhou, L., Hua, M.F., Zhou, Z.J., Tang, G.X., Shen, Z.C., Shou, H.X., 2012. Establishment of methods to rapidly and precisely identify transgenic rice and soybean containing herbicide-resistant gene bar and EPSPS. J. Zhejiang Univ. (Agric. Life Sci.), 38(6):647–654 (in Chinese).
McCabe, D.E., Swain, W.F., Martinell, B.J., Christou, P., 1988. Stable transformation of soybean (Glycine max) by particle acceleration. Nat. Biotechnol., 6(8):923–926. [doi:10.1038/nbt0888-923]
Meurer, C.A., Dinkins, R.D., Collins, G.B., 1998. Factors affecting soybean cotyledonary node transformation. Plant Cell Rep., 18(3–4):180–186. [doi:10.1007/s002990050553]
Monsanto Technology LLC, 2008. Preparation and Use of Plants Embryo Explants for Transformation. US Patent 112628 A2.
Murashige, T., Skoog, F., 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant, 15(3):473–479. [doi:10.1111/j.1399-3054.1962.tb08052.x]
Mysore, K.S., Nam, J., Gelvin, S.B., 2000. An Arabidopsis histone H2A mutant is deficient in Agrobacterium T-DNA integration. PNAS, 97(2):948–953. [doi:10.1073/pnas.97.2.948]
Parrott, W.A., Hoffman, L.M., Hildebrand, D.F., Williams, E.G., Collins, G.B., 1989. Recovery of primary transformants of soybean. Plant Cell Rep., 7(8):615–617. [doi:10.1007/BF00272042]
Paz, M.M., Shou, H.X., Guo, Z.B., Zhang, Z.Y., Banerjee, A.K., Wang, K., 2004. Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica, 136(2): 167–179. [doi:10.1023/B:EUPH.0000030669.75809.dc]
Qiu, L.J., Wang, C.L., Zhou, G.A., Chen, S.Y., Chang, R.Z., 2007. Soybean molecular breeding. Sci. Agric. Sin., 40(11):2418–2436 (in Chinese).
Shou, H.X., Frame, B.R., Whitham, S.A., Wang, K., 2004. Assessment of transgenic maize events produced by particle bombardment or Agrobacterium-mediated transformation. Mol. Breeding, 13(2):201–208. [doi:10.1023/B:MOLB.0000018767.64586.53]
Southern, E.M., 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol., 98(3):503–517. [doi:10.1016/S0022-2836(75)80083-0]
Travella, S., Ross, S.M., Harden, J., Everett, C., Snape, J.W., Harwood, W.A., 2005. A comparison of transgenic barley lines produced by particle bombardment and Agrobacterium-mediated techniques. Plant Cell Rep., 23(12):780–789. [doi:10.1007/s00299-004-0892-x]
Yi, H.C., Sardesai, N., Fujinuma, T., Chan, C.W., Veena, Gelvin, S.B., 2006. Constitutive expression exposes functional redundancy between the Arabidopsis histone H2A gene HTA1 and other H2A gene family members. Plant Cell, 18(7):1575–1589. [doi:10.1105/tpc.105.039719]
Ying, S., He, X.W., Wang, X.R., Shou, H.X., 2008. Assessment of factors affecting the transformation efficiency of soybean cotyledonary-node Agrobacterium-mediated transformation system. Mol. Plant Breeding, 6(1):32–40 (in Chinese).
Zheng, Y., He, X.W., Ying, Y.H., Lu, J.F., Gelvin, S.B., Shou, H.X., 2009. Expression of the Arabidopsis thaliana histone gene AtHTA1 enhances rice transformation efficiency. Mol. Plant, 2(4):832–837. [doi:10.1093/mp/ssp038]
Author information
Authors and Affiliations
Corresponding author
Additional information
The two authors contributed equally to this work
Project supported by the Ministry of Agriculture of China (Nos. 2011ZX08004 and 2009ZX08010013) and the National Natural Science Foundation of China (No. 31172024)
Rights and permissions
About this article
Cite this article
Song, Zy., Tian, Jl., Fu, Wz. et al. Screening Chinese soybean genotypes for Agrobacterium-mediated genetic transformation suitability. J. Zhejiang Univ. Sci. B 14, 289–298 (2013). https://doi.org/10.1631/jzus.B1200278
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1200278