Abstract
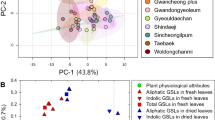
Glucosinolates (GSs) play an important role in plant defense systems and human nutrition. We investigated the content and composition of GSs in the shoots and roots of seven cultivars of pak choi. We found that ‘Si Yue Man’ had the highest total and aliphatic GS contents in the shoots and the highest benzenic GS content in the roots, ‘Shanghai Qing’ contained the highest amounts of benzenic and total GS contents in the roots, while ‘Nanjing Zhong Gan Bai’ had the lowest benzenic, indole, and total GS contents in both the shoots and roots. Therefore, the ‘Si Yue Man’ cultivar appears to be a good candidate for future breeding. Variation between the shoots and roots was also examined, and a significant correlation among the total, aliphatic, and some individual GSs was found, which is of value in agricultural breeding. GS concentrations of the leaf, petiole, and root increased dramatically during the period of rapid growth of the dry matter of the plant 10 to 20 d after transplantation, reaching peak values on Day 20 and decreasing on Day 25. We conclude that the pak choi should be harvested and consumed from 20 to 25 d after transplantation to take advantages of the high GS content in the plant.
Similar content being viewed by others

References
Agerbirk, N., Olsen, C.E., 2012. Glucosinolate structures in evolution. Phytochemistry, 77:16–45. [doi:10.1016/j.phytochem.2012.02.005]
Bellostas, N., Sørensen, J.C., Sørensen, H., 2004. Qualitative and quantitative evaluation of glucosinolates in cruciferous plant during their life cycles. Agroindustria, 3(3):5–10.
Brown, P.D., Tokuhisa, J.G., Reichelt, M., Gershenzon, J., 2003. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry, 62(3):471–481. [doi:10.1016/S0031-9422(02)00549-6]
Brudenell, A.J.P., Griffiths, H., Rossiter, J.T., Baker, D.A., 1999. The phloem mobility of glucosinolates. J. Exp. Bot., 50(335):745–756. [doi:10.1093/jxb/50.335.745]
Castro, A., Aires, A., Rosa, E., Bloem, E., Stulen, I., Kok, L.D., 2004. Distribution of glucosinolates in Brassica oleracea cultivars. Phyt. Ann. Rei Bot., 44(1):133–143.
Chen, S., Andreasson, E., 2001. Update on glucosinolate metabolism and transport. Plant Physiol. Biochem., 39(9):743–758. [doi:10.1016/S0981-9428(01)01301-8]
Chen, S.X., Petersen, B.L., Olsen, C.E., Schulz, A., Halkier, B.A., 2001. Long-distance phloem transport of glucosinolates in Arabidopsis. Plant Physiol., 127(1):194–201. [doi:10.1104/pp.127.1.194]
Chen, X.J., Zhu, Z.J., Gerendas, J., Zimmermann, N., 2008. Glucosinolates in Chinese Brassica campestris vegetables: Chinese cabbage, purple cai-tai, choysum, pakchoi, and turnip. Hortscience, 43(2):571–574.
Clossais-Besnard, N., Larher, F., 1991. Physiological role of glucosinolates in Brassica napus. Concentration and distribution pattern of glucosinolate among plant organs during a complete life cycle. J. Sci. Food Agric., 56(1): 25–38. [doi:10.1002/jsfa.2740560104]
European Community, 1990. Determination of the oilseed glucosinolate content by HPLC. Off. J. Eur. Commun., 170:27–34.
Fahey, J.W., Zalcmann, A.T., Talalay, P., 2001. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry, 56(1):5–51. [doi:10.1016/S0031-9422(00)00316-2]
Griffiths, D.W., Birch, A.N.E., Hillman, J.R., 1998. Antinutritional compounds in the Brassicaceae: analysis, biosynthesis, chemistry and dietary effects. J. Hort. Sci. Biotechnol., 73(1):1–18.
Grubb, C.D., Abel, S., 2006. Glucosinolate metabolism and its control. Trends Plant Sci., 11(2):89–100. [doi:10.1016/j.tplants.2005.12.006]
Halkier, B.A., Gershenzon, J., 2006. Biology and biochemistry of glucosinolates. Ann. Rev. Plant Biol., 57(1):303–333. [doi:10.1146/annurev.arplant.57.032905.105228]
Hanson, P., Yang, R., Chang, L., Ledesma, L., Ledesma, D., 2009. Contents of carotenoids, ascorbic acid, minerals and total glucosinolates in leafy brassica pakchoi (Brassica rapa L. chinensis) as affected by season and variety. J. Sci. Food Agric., 89(5):906–914. [doi:10.1002/jsfa.3533]
He, H., Fingerling, G., Schnitzler, W.H., 2000. Glucosinolate contents and patterns in different organs of Chinese cabbages, Chinese kale (Brassica alboglabra bailey) and choy sum (Brassica campestris L. ssp chinensis var. utilis Tsen et Lee). Angewandte Botanik, 74(1–2):21–25.
Hecht, S.S., 2000. Inhibition of carcinogenesis by isothiocyanates. Drug Metab. Rev., 32(3–4):395–411. [doi:10.1081/DMR-100102342]
Holst, B., Williamson, G., 2004. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep., 21(3):425–447. [doi:10.1039/b204039p]
Kabouw, P., Biere, A., Putten, W.H., van Dam, N.M., 2010. Intra-specific differences in root and shoot glucosinolate profiles among white cabbage (Brassica oleracea var. capitata) cultivars. J. Agric. Food Chem., 58(1):411–417. [doi:10.1021/jf902835k]
Kim, J.K., Sang, M.C., Kim, S.J., Lee, D.J., Lee, S.Y., Lim, S.H., Sun, H.H., Kweon, S.J., Cho, S.H., 2010. Variation of glucosinolates in vegetable crops of Brassica rapa L. ssp. Pekinensis. Food Chem., 119(1):423–428. [doi:10.1016/j.foodchem.2009.08.051]
Kim, Y.S., Milner, J.A., 2005. Targets for indole-3-carbinol in cancer prevention. J. Nutr. Biochem., 16(2):65–73. [doi:10.1016/j.jnutbio.2004.10.007]
Krumbein, A., Schonhof, I., Schreiner, M., 2005. Composition and contents of phytochemicals (glucosinolates, carotenoids and chlorophylls) and ascorbic acid in selected Brassica species (B. juncea, B. rapa subsp. nipposinica var. chinoleifera, B. rapa subsp. Chinensis and B. rapa subsp. rapa). J. Appl. Bot. Food Qual., 79(3):168–174.
Latte, K.P., Appel, K.E., Lampen, A., 2011. Health benefits and possible risks of broccoli—an overview. Food Chem. Toxicol., 49(12):3287–3309. [doi:10.1016/j.fct.2011.08.019]
Malik, M.S., Riley, M.B., Norsworthy, J.K., Bridges, W.J., 2010. Glucosinolate profile variation of growth stages of wild radish (Raphanus raphanistrum). J. Agric. Food Chem., 58(6):3309–3315. [doi:10.1021/jf100258c]
Merritt, S.Z., 1996. Within-plant variation in concentrations of amino acids, sugar, and sinigrin in phloem sap of black mustard, Brassica nigra (L) Koch (Cruciferae). J. Chem. Ecol., 22(6):1133–1145. [doi:10.1007/BF02027950]
Mithen, R.F., Dekker, M., Verkerk, R., Rabot, S., Johnson, I.T., 2000. The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods. J. Sci. Food Agric., 80(7):967–984. [doi:10.1002/(SICI)1097-0010(20000515)80:7〈967::AID-JSFA597〉3.3.CO;2-M]
Nastruzzi, C., Cortesi, R., Esposito E., Menegatti, E., Leoni, O., Iori, R., Palmieri, S., 1996. In vitro cytotoxic activity of some glucosinolate-derived products generated by myrosinase hydrolysis. J. Agric. Food Chem., 44(4):1014–1021. [doi:10.1021/jf9503523]
Padilla, G., Cartea, M.E., Velasco, P., Haro, A., Ordás, A., 2007. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry, 68(4):536–545. [doi:10.1016/j.phytochem.2006.11.017]
Petersen, B.L., Chen, S., Hansen, C.H., Olsen, C.E., Halkier, B.A., 2002. Composition and content of glucosinolates in developing Arabidopsis thaliana. Planta, 214(4):562–571. [doi:10.1007/s004250100659]
Podsedek, A., 2007. Natural antioxidants and antioxidant capacity of Brassica vegetables: a review. LWT-Food Sci. Technol., 40(1):1–11. [doi:10.1016/j.lwt. 2005.07.023]
Potter, M.J., Vanstone, V.A., Davies, K.A., Rathjen, A.J., 2000. Breeding to increase the concentration of 2-phenylethyl glucosinolate in the roots of Brassica napus. J. Chem. Ecol., 26(8):1811–1820. [doi:10.1023/A:1005588405774]
Rosa, E.A.S., Heaney, R.K., Portas, C.A.M., Fenwick, G.R., 1996. Changes in glucosinolate concentrations in Brassica crops (B. oleracea and B. napus) throughout growing seasons. J. Sci. Food Agric., 71(2):237–244. [doi:10.1002/(SICI)1097-0010(199606)71:2〈237::AID-JSFA574〉3.0.CO;2-P]
Schonhof, I., Krumbein, A., Brückner, B., 2004. Genotypic effects on glucosinolates and sensory properties of broccoli and cauliflower. Food, 48(1):25–33. [doi:10.1002/food.200300329]
Smith, T.K., Lund, E.K., Parker, M.L., Clarke, R.G., Johnson, I.T., 2004. Allyl-isothiocyanate causes mitotic block, loss of cell adhesion and disrupted cytoskeletal structure in HT29 cells. Carcinogenesis, 25(8):1409–1415. [doi:10.1093/carcin/bgh149]
Smith, T.K., Lund, E.K., Clarke, R.G., Bennett, R.N., Johnson, I.T., 2005. Effects of Brussels sprout juice on the cell cycle and adhesion of human colorectal carcinoma cells (HT29) in vitro. J. Agric. Food Chem., 53(10):3895–3901. [doi:10.1021/jf048025v]
Tay, D.C.S., Toxopeus, H., 1993. Brassica rapa L. cv. Group Pak Choi in Plant Resources South-East Asia. Number 8: Vegetables. Pudoc Scientific Publishers, Wageningen, p.130–134.
Traka, M., Mithen, R., 2009. Glucosinolates, isothiocyanates and human health. Phytochem. Rev., 8(1):269–282. [doi:10.1007/s11101-008-9103-7]
van Dam, N.M., Tytgat, T.O.G., Kirkegaard, J.A., 2009. Root and shoot glucosinolates: a comparison of their diversity, function and interactions in natural and managed ecosystems. Phytochem. Rev., 8(1):171–186. [doi:10.1007/s11101-008-9101-9]
Velasco, P., Cartea, M.E., Gonzalez, C., Vilar, M., Ordas, A., 2007. Factors affecting the glucosinolate content of kale (Brassica oleracea acephala group). J. Agric. Food Chem., 55(3):955–962. [doi:10.1021/jf0624897]
Verkerk, R., Schreiner, M., Krumbein, A., Ciska, E., Holst, B., Rowland, I., de Schrijver, R., Hansen, M., Gerhauser, C., Mithen, R., et al., 2009. Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res., 53(2):S219–S265. [doi:10.1002/mnfr.200800065]
Vierheilig, H., Bennett, R., Kiddle, G., Kaldorf, M., Ludwig-Muller, J., 2000. Differences in glucosinolate patterns and arbuscular mycorrhizal status of glucosinolate-containing plant species. New Phytol., 146(2):343–352. [doi:10.1046/j.1469-8137.2000.00642.x]
Wittstock, U., Gershenzon, J., 2002. Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol., 5(4):300–307. [doi:10.1016/S1369-5266(02)00264-9]
Yang, J., Zhu, Z.J., Gerendás, J., 2009. Interactive effects of phosphorus supply and light intensity on glucosinolates in pakchoi (Brassica campestris L. ssp. chinensis var. communis). Plant Soil, 323(1–2):323–333. [doi:10.1007/s11104-009-9940-1]
Zangerl, A.R., Bazzaz, F.A., 1993. Theory and Pattern in Plant Defense Allocation. In: Fritz, R.S., Simms, E.L. (Eds.), Plant Resistance to Herbivores and Pathogens. University of Chicago Press, Chicago, p.363–391.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Natural Science Foundation of China (Nos. 30871718 and 31201620), the Zhejiang Provincial Natural Science Foundation of China (No. R3080360), and the Fund for Zhejiang Higher School Innovative Research Team (No. T200916), China
Rights and permissions
About this article
Cite this article
Zhu, B., Yang, J. & Zhu, Zj. Variation in glucosinolates in pak choi cultivars and various organs at different stages of vegetative growth during the harvest period. J. Zhejiang Univ. Sci. B 14, 309–317 (2013). https://doi.org/10.1631/jzus.B1200213
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1200213