Abstract
Objective
To study the oxidative stress and antioxidative response of Cinnamomum camphora seedlings exposed to nitrogen dioxide (NO2) fumigation.
Methods
Measurements were made up of the growth, chlorophyll content, chlorophyll fluorescence, antioxidant system and lipid peroxidation of one-year-old C. camphora seedlings exposed to NO2 (0.1, 0.5, and 4 μl/L) fumigation in open top chambers over a period of 60 d.
Results
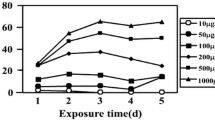
After the first 30 d, 0.5 and 4.0 μl/L NO2 showed insignificant effects on the growth of C. camphora seedlings. However, exposure to 0.5 and 4.0 μl/L NO2 for 15 d significantly reduced their chlorophyll content (P<0.05), enhanced their malondialdehyde (MDA) content and superoxide dismutase (SOD) activity (P<0.05), and also significantly reduced the maximal quantum yield of PSII in the dark [the ratio of variable fluorescence to maximal fluorescence (F v/F m)] (P<0.05). In the latter 30 d, 0.5 μl/L NO2 showed a positive effect on the vitality of the seedlings, which was reflected by a recovery in the ratio of F v/F m and chlorophyll content, and obviously enhanced growth, SOD activity, ascorbate (AsA) content and glutathione reductase (GR) activity (P<0.05); 4.0 μl/L NO2 then showed a negative effect, indicated by significant reductions in chlorophyll content and the ratio of F v/F m, and inhibited growth (P<0.05). Conclusion: The results suggest adaptation of C. camphora seedlings to 60-d exposure to 0.1 and 0.5 μl/L NO2, but not to 60-d exposure to 4.0 μl/L NO2. C. camphora seedlings may protect themselves from injury by strengthening their antioxidant system in response to NO2-induced oxidative stress.
Similar content being viewed by others
References
Ashenden, T.W., 1970. The effects of long-term exposures to SO2 and NO2 pollution on the growth of Dactylis glomerata L. and Poapratensis L. Environ. Pollut., 18(4): 249–258. [doi:10.1016/0013-9327(79)90020-X]
Ashenden, T.W., Bell, S.A., Rafarel, C.R., 1990. Effects of nitrogen dioxide pollution on the growth of three fern species. Environ. Pollut., 66(4):301–318. [doi:10.1016/0269-7491(90)90147-5]
Barnes, J.D., Reiling, K., Davison, A.W., Renner, C.J., 1988. Interaction between ozone and winter stress. Environ. Pollut., 53(1–4):235–254. [doi:10.1016/0269-7491(88)90037-1]
Calatayud, A., Barreno, E., 2001. Chlorophyll a fluorescence, antioxidant enzymes and lipid peroxidation in tomato in response to ozone and benomyl. Environ. Pollut., 115(2): 283–289. [doi:10.1016/S0269-7491(01)00101-4]
Carrasco-Rodriguez, J.L., Valle-Tascon, S.D., 2001. Impact of elevated ozone on chlorophyll a fluorescence in field-grown oat (Avena sativa). Environ. Exp. Bot., 45(2): 133–142. [doi:10.1016/S0098-8472(00)00085-X]
Clyde Hill, A., Bennet, J.H., 1970. Inhibition of apparent photosynthesis by nitrogen oxides. Atmos. Environ., 4(4): 341–348. [doi:10.1016/0004-6981(70)90078-8]
Darrall, N.M., Jäger, H.J., 1984. Biochemical Diagnostic Tests for the Effects of Air Pollution on Plants. In: Koziol, M.J., Whatley, F.R. (Eds.), Gaseous Air Pollutants and Plant Metabolism. Butterworth, London, p.333–350.
Della-Torre, G., Ferranti, F., Lupattelli, M., Pocceschi, N., Figoli, A., Nali, C., Lorenzini, G., 1998. Effects of ozone on morpho-anatomy and physiology of Hedera helix. Chemosphere, 36(4–5):651–656. [doi:10.1016/S0045-6535(97)10102-3]
Dhindsa, R.S., Plumb-Dhindsa, P., Thorpe, T.A., 1981. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J. Exp. Bot., 32(1): 93–101. [doi:10.1093/jxb/32.1.93]
Dixon, R.A., Paiva, N.L., 1995. Stress-induced phenylpropanoid metabolism. The Plant Cell, 7(7):1085–1097. [doi:10.1105/tpc.7.7.1085]
Edjolo, A., Laffray, D., Guerrier, G., 2001. The ascorbate-glutathione cycle in the cytosolic and chloroplastic fractions of drought-tolerant and drought-sensitive poplars. J. Plant Physiol., 158(12):1511–1517. [doi:10.1078/0176-1617-00544]
Ella, E.S., Kawano, N., Ito, O., 2003. Importance of active oxygen-scavenging system in the recovery of rice seedlings after submergence. Plant Sci., 165(1):85–93. [doi: 10.1016/S0168-9452(03)00146-8]
Foyer, C.H., Halliwell, B., 1976. The presence of glutathione reductase in chloroplast: a proposed role in ascorbic acid metabolism. Planta, 133(1):21–25. [doi:10.1007/BF00386001]
Frankart, C., Eullaffroy, P., Vernet, G., 2002. Photosynthetic responses of Lemna minor exposed to xenobiotics, copper, and their combinations. Ecotoxicol. Environ. Safety, 53(3):439–445. [doi:10.1016/S0147-6513(02)00003-9]
Genty, B., Harbinson, J., Briantais, J.M., Baker, N.R., 1990. The relationship between non-photochemical quenching of chlorophyll fluorescence and the rate of photosystem II photochemistry in leaves. Phytosynth. Res., 25(3):249–257. [doi:10.1007/BF00033166]
Guidi, L., Nali, C., Ciompi, S., Lorenzini, G., Soldatini, G.F., 1997. The use of chlorophyll fluorescence and leaf gas exchange as methods for studying the different responses to ozone of two bean cultivars. J. Exp. Bot., 48(1): 173–179. [doi:10.1093/jxb/48.1.173]
Horemans, N., Foyer, C.H., Potters, G., Asard, H., 2000. Ascorbate function and associated transport systems in plants. Plant Physiol. Biochem., 38(7–8):531–540. [doi: 10.1016/S0981-9428(00)00782-8]
Kuźniak, E., Skłodowska, M., 2001. Ascorbate, glutathione and related enzymes in chloroplasts of tomato leaves infected by Botrytis cinerea. Plant Sci., 160(4):723–731. [doi:10.1016/S0168-9452(00)00457-X]
Lee, E.H., Bennett, J.H., 1982. Superoxide dismutase, a possible protective enzyme against ozone injury in snap beans (Phaseolus vulgaris L.). Plant Physiol., 69(6): 1444–1449. [doi:10.1104/pp.69.6.1444]
Lai, Q.X., Bao, Z.Y., Zhu, Z.J., Qian, Q.Q., Mao, B.Z., 2007. Effects of osmotic stress on antioxidant enzymes activities in leaf discs of PSAG12-IPT modified gerbera. J. Zhejiang Univ.-Sci B, 8(7):458–464. [doi:10.1631/jzus.2007.B0458]
Lichtenthaler, H.K., 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology, 148:350–382. [doi:10.1016/0076-6879(87) 48036-1]
Liu, Y.G., Wang, X., Zeng, G.M., Qu, D., Gu, J., Zhou, M., Chai, L.Y., 2007. Cadmium-induced oxidative stress and response of the ascorbate-glutathione cycle in Bechmeria nivea (L.) Gaud. Chemosphere, 69(1):99–107. [doi:10.1016/j.chemosphere.2007.04.040]
Maggs, R., Ashmore, M.R., 1998. Growth and yield responses of Pakistan rice (Oryza sativa L.) cultivars to O3 and NO2. Environ. Pollut., 103(2–3):159–170. [doi:10.1016/S0269-7491(98)00129-8]
Makino, A., Osmond, B., 1991. Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiol., 96(2):355–362. [doi:10.1104/pp.96.2.355]
Marie, B.A., Ormrod, D.P., 1984. Tomato plant growth with continuous exposure to sulphur dioxide and nitrogen dioxide. Environ. Pollut. (Ser. A), 33(3):257–265. [doi:10.1016/0143-1471(84)90015-1]
Maxwell, K., Johnson, G.N., 2000. Chlorophyll fluorescence—a practical guide. J. Exp. Bot., 51(345):659–668. [doi:10.1093/jexbot/51.345.659]
Mehlhorn, H., Óshea, J.M., Wellburn, A.R., 1991. Atmospheric ozone interacts with stress ethylene formation by plants to cause visible plant injury. J. Exp. Bot., 42(1): 17–24. [doi:10.1093/jxb/42.1.17]
Meloni, D.A., Oliva, M.A., Martinez, C.A., Cambraia, J., 2003. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot., 49(1):69–76. [doi:10.1016/S0098-8472(02)00058-8]
Ministry of the Environmental Protection of the People’s Republic of China, 2007. EPA. Available from http://www.sepa.gov.cn/ztbd/sjhjr/2007hjr/tpbd56/200706/P020070625532626111313.pdf [accessed on Jan. 2, 2009] (in Chinese).
Okano, K., Totsuka, T., Fukuzawa, T., Tazaki, T., 1985. Growth responses of plants to various concentrations of nitrogen dioxide. Environ. Pollut. (Ser. A), 38(4):361–373. [doi:10.1016/0143-1471(85)90107-2]
Pan, L.Q., Ren, J.Y., Liu, J., 2006. Responses of antioxidant system and LPO level to benzo(a)pyrene and benzo(k)fluoranthene in the haemolymph of the scallop Chlamys ferrari. Environ. Pollut., 141(3):443–451. [doi: 10.1016/j.envpol.2005.08.069]
Pandey, J., Agrawal, M., 1994. Growth responses of tomato plants to low concentrations of sulphur dioxide and nitrogen dioxide. Scientia Horticulturae, 58(1–2):67–76. [doi:10.1016/0304-4238(94)90128-7]
Pleijel, H., Skärby, L., Ojanperä, K., Selldén, G., 1994. Exposure of oats, Avena sativa L., to filtered and unfiltered air in open-top chambers: effects on grain yield and quality. Environ. Pollut., 86(2):129–134. [doi:10.1016/0269-7491(94)90183-X]
Potters, G., Gara, L.D., Asard, H., Horemans, N., 2002. Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol. Biochem., 40(6–8): 537–548. [doi:10.1016/S0981-9428(02)01414-6]
Price, A., Lucas, P.W., Lea, P.J., 1990. Age dependent damage and glutathione metabolism in ozone fumigated barley: a leaf section approach. J. Exp. Bot., 41(10):1309–1317. [doi:10.1093/jxb/41.10.1309]
Qiao, Z., Murray, F., 1998. The effects of NO2 on the uptake and assimilation of nitrate by soybean plants. Environ. Exp. Bot., 39(1):33–40. [doi:10.1016/S0098-8472(97)00023-3]
Ra, H.S.Y., Geiser, L.H., Crang, R.F.E., 2005. Effects of season and low-level air pollution on physiology and element content of lichens from the U.S. Pacific Northwest. Sci. Total Environ., 343(1–3):155–167. [doi:10.1016/j.scitotenv.2004.10.003]
Rai, V., Vajpayee, P., Singh, S.N., Mehrotra, S., 2004. Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci., 167(5):1159–1169. [doi:10.1016/j.plantsci.2004.06.016]
Ramge, P., Badeck, F.W., Plochl, M., 1993. Apoplastic antioxidants as decisive elimination factors within the uptake process of nitrogen dioxide into leaf tissues. New Phytol., 125(4):771–785. [doi:10.1111/j.1469-8137.1993.tb03927.x]
Rice-Evans, C.A., Miller, N.J., Paganga, G., 1996. Structure-antioxidant activity relationship of flavonoids and phenolic acids. Free Radic. Biol. Med., 20(7):933–956. [doi:10.1016/0891-5849(95)02227-9]
Sabaratnam, S., Gupat, G., 1988. Effects of nitrogen dioxide on biochemical and physiological characteristics of soybean. Environ. Pollut., 55(2):149–158. [doi:10.1016/0269-7491(88)90125-X]
Sabaratnam, S., Gupat, G., Mulchi, C., 1988. Effects of nitrogen dioxide on leaf chlorophyll and nitrogen content of soybean. Environ. Pollut., 51(2):113–120. [doi:10.1016/0269-7491(88)90200-X]
Sakaki, T., Kondo, N., Sugahara, K., 1983. Breakdown of photosynthetic pigments and lipids in spinach leaves with ozone fumigation: role of active oxygens. Physiol. Plantarum, 59(1):28–34. [doi:10.1111/j.1399-3054.1983.tb06566.x]
Shalata, A., Tal, M., 1998. The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol. Plant., 104(2):169–174. [doi:10.1034/j.1399-3054.1998.1040204.x]
Shimazaki, K., Yu, S.W., Sakaki, T., Tanaka, K., 1992. Differences between spinach and kidney bean plants in terms of sensitivity to fumigation with NO2. Plant Cell Physiol., 33(3):267–273.
Smirnoff, N., 1996. The function and metabolism of ascorbic acid in plants. Ann. Bot., 78(6):661–669. [doi:10.1006/anbo.1996.0175]
Takahashi, M., Higaki, A., Nohno, M., Kamada, M., Okamura, Y., Matsui, K., Kitani, S., Morikawa, H., 2005. Differential assimilation of nitrogen dioxide by 70 taxa of roadside trees at an urban pollution level. Chemosphere, 61(5): 633–639. [doi:10.1016/j.chemosphere.2005.03.033]
Tian, D.L., Fu, X.P., Fang, X., Xiang, W.H., 2007. Effect of simulated acid rain on photosynthetic characteristics in Cinnamomum camphora seedlings. Scientia Silvae Sinicae, 43:29–35 (in Chinese).
Walmsley, L., Ashmore, M.R., Bell, J.N.B., 1980. Adaptation of radish Raphanus sativus L. in response to continuous exposure to ozone. Environ. Pollut. (Ser. A), 23(3):165–177. [doi:10.1016/0143-1471(80)90044-6]
Wu, Y.X., Tiedemann, A., 2002. Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ. Pollut., 116(1):37–47. [doi:10.1016/S0269-7491(01)00174-9]
Yu, S.W., Li, L., Shimazaki, K., 1988. Response of spinach and kidneybean plants to nitrogen dioxide. Environ. Pollut., 55(1):1–13. [doi:10.1016/0269-7491(88)90155-8]
Zeevaart, A.J., 1976. Some effects of fumigation plants for short periods with NO2. Environ. Pollut., 11(2):97–108. [doi:10.1016/0013-9327(76)90022-7]
Zhang, L.L., Lin, Y.M., 2008. Tannins from Canarium album with potent antioxidant activity. J. Zhejiang Univ.-Sci. B, 9(5):407–415. [doi:10.1631/jzus.B0820002]
Zheng, W.J., 1983. Chinese Tree Records. Volume 1, Chinese Forestry Press, Beijing, China, p.749 (in Chinese).
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by Zhejiang Keystone Projects (No. 2005C22056), and the Zhejiang Provincial Natural Science Foundation of China (No. Y5080011)
Rights and permissions
About this article
Cite this article
Chen, Zm., Chen, Yx., Du, Gj. et al. Effects of 60-day NO2 fumigation on growth, oxidative stress and antioxidative response in Cinnamomum camphora seedlings. J. Zhejiang Univ. Sci. B 11, 190–199 (2010). https://doi.org/10.1631/jzus.B0910350
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B0910350
Key words
- Cinnamomum camphora
- Fumigation
- Growth
- Chlorophyll content
- Chlorophyll fluorescence
- Antioxidant
- Lipid peroxidation