Abstract
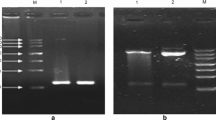
Bee venom phospholipase A2 (BvPLA2) is a lipolytic enzyme that catalyzes the hydrolysis of the sn-2 acyl bond of glycerophospholipids to liberate free fatty acids and lysophospholipids. In this work, a new BvPLA2 (AccPLA2) gene from the Chinese honeybee (Apis cerana cerana) venom glands was inserted into bacmid to construct a recombinant transfer vector. Tn-5B-4 (Tn) cells were transfected with the recombinant bacmid DNA for expression. Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis revealed a double band with molecular weights of 16 and 18 kDa. Products of hexahistidine AccPLA2 fusion protein accumulated up to 5.32% of the total cellular proteins. The AccPLA2 fusion protein was cross reactive with the anti-AmPLA2 (BvPLA2 of the European honeybee, Apis mellifera) polyclonal serum. The reaction resulted in a double glycosylation band, which agrees with the band generated by the native AmPLA2 in Western blot analysis. The PLA2 activity of the total extracted cellular protein in the hydrolyzing egg yolk is about 3.16 μmol/(min·mg). In summary, the recombinant AccPLA2 protein, a native BvPLA2-like structure with corresponding biological activities, can be glycosylated in Tn cells. These findings provided fundamental knowledge for potential genetic engineering to produce AccPLA2 in the pharmaceutical industry.
Similar content being viewed by others
References
Altmann, F., Kubelka, V., Staudacher, E., Uhl, K., Marz, L., 1991. Characterization of the isoforms of phospholipase A2 from honeybee venom. Insect Biochem., 21(5):467–472. [doi:10.1016/0020-1790(91)90099-Z]
Altmann, F., Staudacher, E., Wilson, I.B.H., Marz, L., 1999. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconjugate J., 16(2):109–123. [doi:10.1023/A:1026488408951]
Annand, R.R., Kontoyianni, M., Penzotti, J.E., Dudler, T., Lybrand, T.P., Gelb, M.H., 1996. Active site of bee venom phospholipase A2: the role of histidine-34, As-partate-64 and Tyrosine-87. Biochemistry, 35(14):4591–4601. [doi:10.1021/bi9528412]
Balsinde, J., Winstead, M.V., Dennis, E.A., 2002. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett., 531(1):2–6. [doi:10.1016/S0014-5793(02)03413-0]
Dennis, E.A., 1994. Diversity of group types, regulation and function of phospholipase A2. J. Biochem., 269(18):13057–13060.
Dennis, E.A., 1997. A growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem. Sci., 22(1):1–2. [doi:10.1016/S0968-0004(96)20031-3]
Dudler, T., Chen, W.Q., Wang, S.S., Schneider, T., Annand, R.R., Dempcy, R.O., Crameri, R., Gmachl, M., Suter, M., Gelb, M.H., 1992. High-level expression in Escherichia coli and rapid purification of enzymatically active honey bee venom phospholipase A2. Biochim. Biophys. Acta, 1165(2):201–210.
Fenard, D., Lambeau, G., Valentin, E., Lefebvre, J.C., Lazdunski, M., Doglio, A., 1999. Secreted phospholipases A2, a new class of HIV inhibitors that block virus entry into host cells. J. Clin. Invest., 104(5):611–618. [doi:10.1172/JCI6915]
Fenard, D., Lambeau, G., Maurin, T., Lefebvre, J.C., Doglio, A., 2001. A peptide derived from bee venom-secreted phospholipase A2 inhibits replication of T-cell tropic HIV-1 strains via interaction with the CXCR4 chemokine receptor. Mol. Pharmacol., 60(2):341–347.
Habermann, E., 1972. Bee and wasp venoms. Science, 177(4046):314–322. [doi:10.1126/science.177.4046.314]
King, T.P., Spangfort, M.D., 2000. Structure and biology of stinging insect venom allergens. Int. Arch. Allergy Imm., 123(2):99–106. [doi:10.1159/000024440]
Kubelka, V., Almann, F., Staudacher, E., Tretter, V., Marz, L., Hard, K., Kamerling, J.P., Vliegenthart, J.F., 1993. Primary structures of the N-linked carbohydrate chains from honeybee venom phospholipase A2. Eur. J. Biochem., 213(3):1193–1204. [doi:10.1111/j.1432-1033.1993.tb17870.x]
Kuchler, K., Gmachl, M., Sippl, M.J., Krell, G., 1989. Analysis of the cDNA for phospholipase A2 from honeybee venom glands-the deduced amino acid sequence reveals homology to the corresponding vertebrate enzymes. Eur. J. Biochem., 184(1):249–254. [doi:10.1111/j.1432-1033.1989.tb15014.x]
Li, J.H., Zhang, C.X., Shen, L.R., Tang, Z.H., Cheng, J.A., 2005. Expression and regulation of phospholipase A2 in venom gland of the Chinese honeybee, Apis cerana cerana. Arch. Insect Biochem. Physiol., 60(1):1–12. [doi:10.1002/arch.20075]
Luckow, V.A., Lee, S.C., Barry, G.F., Olins, P.O., 1993. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J. Virol., 67(8):4566–4579.
Mingarro, I., Prez-Paya, E., Pinilla, C., Appel, J.R., Houghten, R.A., Blondelle, S.E., 1995. Activation of bee venom phospholipase A2 through a peptide-enzyme complex. FEBS Lett., 372(1):131–134. [doi:10.1016/0014-5793(95)00964-B]
Mukherjee, A.B., Miele, L., Pattabiraman, N., 1994. Phospholipases A2 enzymes: regulation and hysiological role. Biochem. Pharmacol., 48(1):1–10. [doi:10.1016/0006-2952(94)90216-X]
Murakami, M., Kudo, I., 2002. Phospholipase A2. J. Biochem., 131(2):285–292.
Nakashima, S., Kitamoto, K., Arioka, M., 2004. The catalytic activity, but not receptor binding, of PLA2s plays a critical role for neurite outgrowth induction in PC12 cells. Brain Res., 1015(1/2):207–211. [doi:10.1016/j.brainres.2004.04.069]
Oldroyd, B.P., Wongsiri, S., 2006. Asian Honeybees. Biology, Conservation, and Human Interactions. Harvard University Press, Cambridge, Massachusetts, USA, p.36–64.
Owen, M.D., Pfaff, L.A., Reisman, R.E., Wypych, J., 1990. Phospholipase A2 in venom extracts from honey bees (Apis mellifera L.) of different ages. Toxicon, 28(7):813–820. [doi:10.1016/S0041-0101(09)80004-4]
Palomares, L.A., Joosten, C.E., Hughes, P.R., Granados, R.R., Shuler, M.L., 2003. Novel insect cell line capable of complex N-glycosylation and sialylation of recombinant proteins. Biotechnol. Prog., 19(1):185–192. [doi:10.1021/bp025598o]
Rodriguez de Turco, E.B., Jackson, F.R., DeCoster, M.A., Kolko, M., Bazan, N.G., 2002. Glutamate signalling and secretory phospholipase A2 modulate the release of arachidonic acid from neuronal membranes. J. Neurosci. Res., 68(5):558–567. [doi:10.1002/jnr.10239]
Sambrook, J., Russell, D.W., 2002. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, New York, USA.
Schmidt, J.O., 1995. Toxinology of venoms from the honeybee genus Apis. Toxicon, 33(7):917–927. [doi:10.1016/0041-0101(95)00011-A]
Scott, D.L., White, S.P., Otwinowski, Z., Yuan, W., Gelb, M.H., Sigler, P.B., 1990a. Interfacial catalysis: the mechanism of phospholipase. Science, 250(4987):1541–1546. [doi:10.1126/science.2274785]
Scott, D.L., Otwinowski, Z., Gelb, M.H., Sigler, P.B., 1990b. Crystal structure of bee-venom phospholipase A2 in a complex with a transition-state analogue. Science, 250(4987):1563–1566. [doi:10.1126/science.2274788]
Shang, J.Y., Shao, Y.M., Lang, G.J., Yuan, G., Tang, Z.H., Zhang, C.X., 2007. Expression of two types of acetylcholinesterase gene from the silkworm, Bombyx mori, in insect cells. Insect Sci., 14(6):443–449.
Shen, L.S., Zhang, C.Z., Cheng, J.A., 2002. Cloning and sequencing of genes encoding phospholipase A2 from the venom of Apis cerana cerana and A. mellifera. J. Agric. Biotechnol., 10(1):29–32 (in Chinese).
Shipolini, R.A., Callewaert, G.L., Cottrell, R.C., Vernon, C.A., 1974a. The amino-acid sequence and carbohydrate content of phospholipase A2 from bee venom. Eur. J. Biochem., 48(2):465–476. [doi:10.1111/j.1432-1033.1974.tb03787.x]
Shipolini, R.A., Doonan, S., Vernon, C.A., 1974b. The disulphide bridges of phospholipase A2 from bee venom. Eur. J. Biochem., 48(2):477–483. [doi:10.1111/j.1432-1033.1974.tb03788.x]
Smith, G.E., Summers, M.D., Fraser, M., 1983. Production of human belta interferon in insect cell with baculovirus expression vectors. J. Mol. Cell. Biol., 3(12):2156–2165.
Soldatova, L.N., Crameri, R., Gmachl, M., Kemeny, D.M., Schmidt, M., Weber, M., Mueller, U.R., 1998. Superior biologic activity of the recombinant bee venom allergen hyaluronidase expressed in baculovirus infected insect cells as compared with Escherichia coli. J. Allergy Clin. Immunol., 101(5):691–698. [doi:10.1016/S0091-6749(98)70179-4]
Toki, D., Sarkar, M., Yip, B., Reck, F., Joziasse, D., Fukuda, M., Schachter, H., Brockhausen, I., 1997. Expression of stable human O-glycan core 2β-1,6-N-acetylglucosaminyltransferase in Sf9 insect cells. Biochem. J., 325(1):63–69.
Whitfield, C.W., Behura, S.K., Berlocher, S.H., Clark, A.G.J., Johnston, S., Sheppard, W.S., Smith, D.R., Suarez, A.V., Weaver, D., Tsutsui, N.D., 2006. Thrice out of Africa: ancient and recent expansions of the honey bee, Apis mellifera. Science, 314(5799):642–645. [doi:10.1126/science.1132 772]
Xu, P., Shi, M., Chen, X.X., 2009. Antimicrobial peptide evolution in the Asiatic honey bee Apis cerana. PLoS ONE, 4(1):e4239. [doi:10.1371/journal.pone.0004239]
Zhang, C.X., Tang, X.D., Cheng, J.A., 2008. The utilization and industrialization of insect resources in China. Entomol. Res., 38(S1):S38–S47. [doi:10.1111/j.1748-5967.2008.00173.x]
Zhao, M., Brunk, U.T., Eaton, J.W., 2001. Delayed oxidant-induced cell death involves activation of phospholipase A2. FEBS Lett., 509(3):399–404. [doi:10.1016/S0014-5793(01)03184-2]
Author information
Authors and Affiliations
Corresponding author
Additional information
Project (No. 2007AA10Z324) supported by the National High-Tech Research and Development Program (863) of China
Rights and permissions
About this article
Cite this article
Shen, Lr., Ding, Mh., Zhang, Lw. et al. Expression of a bee venom phospholipase A2 from Apis cerana cerana in the baculovirus-insect cell. J. Zhejiang Univ. Sci. B 11, 342–349 (2010). https://doi.org/10.1631/jzus.B0900254
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B0900254