Abstract
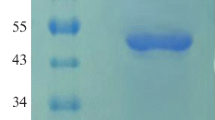
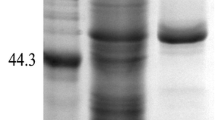
The phyA m gene encoding acid phytase and optimized neutral phytase phyCs gene were inserted into expression vector pPIC9K in correct orientation and transformed into Pichia pastoris in order to expand the pH profile of phytase and decrease the cost of production. The fusion phytase phyA m-phyCs gene was successfully overexpressed in P. pastoris as an active and extracellular phytase. The yield of total extracellular fusion phytase activity is (25.4±0.53) U/ml at the flask scale and (159.1±2.92) U/ml for high cell-density fermentation, respectively. Purified fusion phytase exhibits an optimal temperature at 55 °C and an optimal pH at 5.5∼6.0 and its relative activity remains at a relatively high level of above 70% in the range of pH 2.0 to 7.0. About 51% to 63% of its original activity remains after incubation at 75 °C to 95 °C for 10 min. Due to heavy glycosylation, the expressed fusion phytase shows a broad and diffuse band in SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). After deglycosylation by endoglycosidase H (EndoHf), the enzyme has an apparent molecular size of 95 kDa. The characterization of the fusion phytase was compared with those of phyCs and phyA m.
Similar content being viewed by others
References
André, N., Cherouati, N., Prual, C., Steffan, T., Zeder-Lutz, G., Magnin, T., Pattus, F., Michel, H., Wagner, R., Reinhart, C., 2006. Enhancing functional production of G protein-coupled receptors in Pichia pastoris to levels required for structural studies via a single expression screen. Protein Science, 15(5):1115–1126. [doi:10.1110/ps.062098206]
Applegate, T.J., Webel, D.M., Lei, X.G., 2003. Efficacy of a phytase derived from Escherichia coli and expressed in yeast on phosphorus utilization and bone mineralization in turkey poults. Poultry Science, 82(11):1726–1732.
Augspurger, N.R., Webel, D.M., Lei, X.G., Baker, D.H., 2003. Efficacy of an E. coli phytase expressed in yeast for releasing phytate-bound phosphorus in young chicks and pigs. J. Anim. Sci., 81(2):474–483.
Bitar, K., Reinhold, J.G., 1972. Phytase and alkaline phosphatase activities in intestinal mucosae of rat, chicken, calf, and man. Biochem. Biophys. Acta, 268(2):442–452.
Bohn, L., Meyer, A.S., Rasmussen, S.K., 2008. Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ. Sci. B, 9(3):165–191. [doi:10.1631/jzus.B0710640]
Chen, C.C., Wu, P.H., Huang, C.T., Cheng, K.J., 2004. A Pichia pastoris fermentation strategy for enhancing the heterologous expression of an Escherichia coli phytase. Enzyme and Microbial Technology, 35(4):315–320. [doi:10.1016/j.enzmictec.2004.05.007]
Chen, H., Zhao, H.X., Wang, H.N., 2003. The research of phytase’s characteristies from gene engineering yeast. Microbiology, 30(3):38–41 (in Chinese).
Chen, H., Zhao, H.X., Wang, H.N., 2005. Increasing expression level of phytase gene (phyA) in Pichia pastoris by changing rare codons. Chinese Journal of Biochemistry and Molecular Biology, 21(2):171–175 (in Chinese).
Common, F.H., 1989. Biological availability of phosphorus for pigs. Nature, 143:370–380.
Cregg, J.M., Higginns, D.R., 1995. Production of foreign proteins in the yeast Pichia pastoris. Can. J. Bot., 73(Suppl.):5891–5987.
Han, Y., Lei, X.G., 1999. Role of glycosylation in the functional expression of an Aspergillus niger phytase (phyA) in Pichia pastoris. Archives of Biochemistry and Biophysics, 364(1):83–90. [doi:10.1006/abbi.1999.1115]
Han, Y., Wilson, D.B., Lei, X.G., 1999. Expression of an Aspergillus niger phytase gene (phyA) in Saccharomyces cerevisiae. Applied and Environmental Microbiology, 65(5):1915–1918.
Janne, K., Marko, L., Paivi, N., Nisse, K., Juha, A., 1998. Isolation, characterization, molecular gene cloning, and sequencing of a novel phytase from Bacillus subtilis. Applied and Environmental Microbiology, 64(6):2079–2085.
Kim, T., Mullaney, E.J., Porres, J.M., Roneker, K.R., Crowe, S., Rice, S., Ko, T., Ullah, A.H.J., Daly, C.B., Welch, R., Lei, X.G., 2006. Shifting the pH profile of Aspergillus niger phyA phytase to match the stomach pH enhances its effectiveness as an animal feed additive. Applied and Environmental Microbiology, 72(6):4397–4403 [doi:10.1128/AEM.02612-05]
Kim, T.W., Lei, X.G., 2005. An improved method for a rapid determination of phytase activity in animal feed. J. Anim. Sci., 83:1062–1067.
Kim, Y.O., Lee, J.K., Kim, H.K., Yu, J.H., Oh, T.K., 1998. Cloning of the thermostable phytase gene (phy) from Bacillus sp. DS11 and its expression in E. coli. FEMS Microbiology Letters, 162(1):185–191. [doi:10.1111/j.1574-6968.1998.tb12997.x]
Kim, Y.O., Lee, J.K., Oh, B.C., Oh, T.K., 1999. High level expression of a recombinant thermostable phytase in Bacillus subtilis. Biosci. Biotechnol. Biochem., 63(12):2205–2207. [doi:10.1271/bbb.63.2205]
Liu, B.L., Rafiq, A., Tzeng, Y.M., Rob, A., 1998. The induction and characterization of phytase and beyond. Enzyme and Microbial Technology, 22(5):415–424. [doi:10.1016/S0141-0229(97)00210-X]
Markus, W., Luis, P., Arno, F., Roland, R., Michek, T., Alexandra, K., Anke, M., Martin, L., Line, S., Ursro, T., et al., 1999. Biophysical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): molecular size, glycosylation pattern, and engineering of proteolytic resistance. Applied and Environmental Microbiology, 65(2):359–366.
Olukosi, O.A., Sands, J.S., Adeola, O., 2007. Supplementation of carbohydrases or phytase individually or in combination to diets for weanling and growing-finishing pigs. J. Anim. Sci., 855(7):1702–1711. [doi:10.2527/jas.2006-709]
Reddy, N.R., Sathe, S.K., Salunkhe, D.K., 1982. Phytates in legumes and cereals. Adv. Food Res., 28:1–92.
Rodriguez, E., Han, Y., Lei, X.G., 1999. Cloning, sequencing, and expression of an Escherichia coli acid phosphatase/phytase gene (appA2) isolated from pig colon. Biochem. Biophys. Res. Commun., 257(1):117–123. [doi:10.1006/bbrc.1999.0361]
Rodriguez, E., Mullaney, E.J., Lei, X.G., 2000. Expression of the Aspergillus fumigatus phytase gene in Pichia pastoris and characterization of the recombinant enzyme. Biochemical and Biophysical Research Communications, 268(2):373–378. [doi:10.1006/bbrc.2000.2121]
Romanos, M., 1995. Advances in the use of Pichia pastor is for high-level expression. Curr. Opin. Biotechnol., 6(5):527–533. [doi:10.1016/0958-1669(95)80087-5]
Rutherfurd, S.M., Chung, T.K., Moughan, P.J., 2002. The effect of microbial phytase on ileal phosphorus and amino acid digestibility in the broiler chicken. Br. Poultry Sci., 43(4):598–606. [doi:10.1080/0007166022000004516]
Tye, A.J., Siu, F.K.Y., Leung, T.Y.C., Lim, B.L., 2002. Molecular cloning and the biochemical characterization of two novel phytases from B. subtilis 168 and B. licheniformis. Appl. Microbiol. Biotechnol., 59(2):190–197. [doi:10.1007/s00253-002-1033-5]
Veum, T.L., Bollinger, D.W., Buff, C.E., Bedford, M.R., 2006. A genetically engineered Escherichia coli phytase improves nutrient utilization, growth performance, and bone strength of young swine fed diets deficient in available phosphorus. J. Anim. Sci., 84:1147–1158.
Vuolanto, A., von Weymarn, N., Kerovuo, J., Ojamo, H., Leisola, M., 2001. Phytase production by high cell density culture of recombinant Bacillus subtilis. Biotechnology Letters, 23(10):761–766. [doi:10.1023/A:101036932558]
Wang, H.N., Wu, Q., Zhao, H.X., Zou, L.K., Liu, S.G., 2005. Secretory expression of Bacillus subtilis phytase phyC in Pichia pastoris. Journal of Zheijiang University (Agric. & Life Sci.), 31(5):621–627 (in Chinese).
Wodzinski, R.J., Ullah, A.H., 1996. Phytase. Adv. Appl. Microbiol., 42:263–302.
Wu, Q., Wang, H.N., Zhou, L.K., Zhao, H.X., Liu, S.G., 2004a. Expression of phytase phyC from B. subtilis in E. coli and its effect in enzymetic thermostability. High Technology Letters, 14(5):23–27 (in Chinese).
Wu, Q., Wang, H.N., Zou, L.K., Zhao, H.X., Liu, S.G., 2004b. Intrcellular expression of Bacillus subtilis phytase phyC in Pichia patoris. Journal of Jishou University (Natural Science Edition), 25(1):36–41 (in Chinese).
Xie, Z.W., Wang, H.N., 2007. Preliminary study on flask fermentation condition of producing phytase by engineering bacterium. China Feed, 3:13–16 (in Chinese).
Xiong, A.S., Yao, Q.H., Peng, R.H., Han, P.L., Cheng, Z.M., Li, Y., 2005. High level expression of a recombinant acid phytase gene in Pichia pastoris. Journal of applied Microbiology, 98(2):418–428. [doi:10.1111/j.1365-2672.2004.02476.x]
Yoshimasu, M.A., Tanaka, T., Ahn, J.K., Yada, R.Y., 2004. Effect of N-linked glycosylation on the aspartic proteinase poreine pepsin expressed from Pichia pastoris. Glycobiology, 14(5):417–429. [doi:10.1093/glycob/cwh024]
Zhang, W., Mullaney, E.J., Lei, X.G., 2007. Adopting selected hydrogen bonding and ionic interactions from Aspergillus fumigatus phytase structure improves the thermostability of Aspergillus niger phyA phytase. Applied and Environmental Microbiology, 73(9):3069–3076. [doi:10.1128/AEM.02970-06]
Zinin, N.V., Serkina, A.V., Gelfand, M.S., Shevelev, A.B., Sineoky, S.P., 2004. Gene cloning, expression and characterization of novel phytase from Obesumbacterium proteus. FEMS Microbiology Letters, 236(2):283–290. [doi:10.1111/j.1574-6968.2004.tb09659.x]
Zou, L.K., Wang, H.N., Pan, X., Xie, T., Wu, Q., Xie, Z.W., Zhou, W.R., 2006. Design and expression of s synthetic phyC gene encoding the neutral phytase in Pichia pastoris. Acta Biochimica et Biophysica Sinica, 38(11):803–811. [doi:10.1111/j.1745-7270.2006.00231.x]
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Key Technologies R & D Program of China during the 10th Five-Year Plan Period (No. 2002BA514A-12), the Education Department of Sichuan Province (No. 2006B014) and the innovative Fund for Distinguished Young Scholars of Sichuan Agricultural University, China
Rights and permissions
About this article
Cite this article
Zou, Lk., Wang, Hn., Pan, X. et al. Expression, purification and characterization of a phyA m-phyCs fusion phytase. J. Zhejiang Univ. Sci. B 9, 536–545 (2008). https://doi.org/10.1631/jzus.B0720006
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B0720006