Abstract
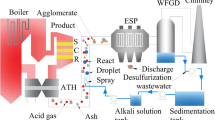
HCl in coal-fired flue gas has adverse impact on the environment, equipment, and the flue gas desulfurization (FGD) system. The existence of HCl also increases the difficulty of the treatment of desulfurization waste water. Semi-dry dechlorination technology is put forward to attach chlorine to fly ash by spraying in alkaline solution. Simultaneously, desulphurization waste water is used as the solvent of alkali, and this could help realize the target of near-zero emission of desulfurization waste water. CHEMKIN is used to build a chemical kinetics model, which is based on the measured components of flue gas in a coal-fired power plant. NaOH is set as the alkali absorbent in the model. Both the competitive relationship of SO2 and HCl and the effects of different factors on HCl reaction efficiency are analyzed. SO2 with high concentration would compete for more NaOH, but when Na/Cl (ratio in mole) is 1, the reaction efficiency of HCl achieves 22.28%, and it is positively correlated with Na/Cl. When Na/Cl surpasses 5, the reaction efficiency of HCl increases to beyond 70%. As Na/Cl continues to increase, there is a slower growth of HCl reaction efficiency and it finally achieves 100% when Na/Cl reaches 12. With a fixed value of Na/Cl, a change of 1000 mg/m3 in SO2 concentration would change the reaction efficiency of HCl about 13%. The effect of flue temperature on HCl reaction efficiency is not significant. Acid gases in flue gas react with NaOH completely in 0.1 s and come to equilibrium after about 1 s.
概要
目的
燃煤锅炉烟气中的HCl 对环境、设备及脱硫系统有不利的影响,也是脱硫废水处理的难点。本文提出半干法脱氯技术,探讨其技术合理性,并研究不同因素对反应效率的影响,为进一步工程应用提供理论依据,实现燃煤烟气中HCl 的脱除及脱硫废水零排放。
创新点
1. 提出半干法脱氯技术,将氯离子固化到飞灰中;2. 脱氯后脱硫废水大幅减少,将脱硫废水作为碱基溶剂回喷到烟道中,实现脱硫废水零排放;3.建立良搅拌反应器(PSR)模型,探讨半干法脱氯过程的化学动力学反应机理与关键参数。
方法
1. 通过CHEMKIN 构建PSR 模型,模拟实际燃煤烟气组分下的半干法脱氯过程;2. 通过敏感性分析,探讨SO2 与HCl 之间的竞争关系(图8~10);3. 通过单变量模拟,研究不同因素对反应效率的影响(图13~16)
结论
1. 烟气中的SO2 对HCl 的脱除存在较大竞争关系,但即使Na/Cl(摩尔比)为1 时,HCl 的反应效率依然可观。2. HCl 的反应效率与Na/Cl 呈正相关;综合考虑反应效率和运行成本,设置Na/Cl为5 较为合理。3. SO2 浓度的小范围变化不会对HCl 的反应效率造成显著影响。4. 仅通过化学动力学模拟,烟气温度对HCl 的反应效率影响不明显。5. 烟气中酸性气体与NaOH 在0.1 s 内即可完成反应,在1 s 左右达到平衡。
Similar content being viewed by others
References
Ayotte P, Hébert M, Marchand P, 2005. Why is hydrofluoric acid a weak acid? The Journal of Chemical Physics, 123(18):184501. https://doi.org/10.1063/1.2090259
Deng S, Zhang C, Liu Y, et al., 2014. A full-scale field study on chlorine emission of pulverized coal-fired power plants in China. Research of Environmental Sciences, 27(2):127–133.
Farmer RW, Jarvis JB, Moser R, 1989. Effects of aluminum fluoride chemistry in wet limestone flue-gas desulfurization. Chemical Engineering Communications, 77(1):135–154. https://doi.org/10.1080/00986448908940177
Finkelman RB, Belkin HE, Zheng BS, 1999. Health impacts of domestic coal use in China. Proceedings of the National Academy of Sciences, 96(7):3427–3431. https://doi.org/10.1073/pnas.96.7.3427
Gao X, Huo W, Luo ZY, et al., 2008. CFD simulation with enhancement factor of sulfur dioxide absorption in the spray scrubber. Journal of Zhejiang University-SCIENCE A, 9(11):1601–1613. https://doi.org/10.1631/jzus.A0820507
Glarborg P, Marshall P, 2005. Mechanism and modeling of the formation of gaseous alkali sulfates. Combustion and Flame, 141(1–2):22–39. https://doi.org/10.1016/j.combustflame.2004.08.014
Hall B, Sehager P, Lindqvis O, 1991. Chemical reactions of mercury in combustion flue gases. Water Air & Soil Pollution, 56(1):3–14. https://doi.org/10.1007/BF00342256
Hartman M, Svoboda K, Pohorely M, et al., 2014. Reaction of hydrogen chloride gas with sodium carbonate and its deep removal in a fixed-bed reactor. Industrial and Engineering Chemistry Research, 53(49):19145–19158. https://doi.org/10.1021/ie503480k
Hu S, Ding SF, Fan ZS, 2015. Zero release technology of desulfurization waste water in coal-fired power plant. Clean Coal Technology, 21(2):129–133 (in Chinese).
Jimenez A, Martinez-Tarazona MR, Suarez-Ruiz I, 2000. The mode of occurrence and origin of chlorine in Puertollano coals (Spain). Fuel and Energy Abstracts, 41(2):73. https://doi.org/10.1016/S0140-6701(00)90593-9
Liu ZS, Wey MY, Lin CL, 2002. Reaction characteristics of Ca(OH)2, HCl and SO2 at low temperature in a spray dryer integrated with a fabric filter. Journal of Hazardous Materials, 95(3):291–304. https://doi.org/10.1016/S0304-3894(02)00142-5
Mori T, Matsuda S, Nakajima F, et al., 1981. Effect of Al3+ and F on desulfurization reaction in the limestone slurry scrubbing process. Industrial and Engineering Chemistry Process Design and Development, 20(1):144–147. https://doi.org/10.1021/i200012a022
Pang YK, 1998. Modern Drying Technology. Chemical Industry Press, Beijing, China, p.262–282 (in Chinese).
Poskrobko S, Lach J, Krol D, 2010. Experimental investigation of hydrogen chloride bonding with calcium hydroxide in the furnace of a stoker-fired boiler. Energy and Fuels, 24(3):1948–1957. https://doi.org/10.1021/ef901534d
Poskrobko S, Krol D, Lach J, 2012. Hydrogen chloride bonding with calcium hydroxide in combustion and two-stage combustion of fuels from waste. Energy and Fuels, 26(2): 842–853. https://doi.org/10.1021/ef2016599
Shao DK, Hutchinson EJ, Cao H, et al., 1994. Behavior of chlorine during coal pyrolysis. Energy and Fuels, 8(2): 399–401. https://doi.org/10.1021/ef00044a017
Stein J, Kind M, Schlünder EU, 2002. The influence of HCl on SO2 absorption in the spray dry scrubbing process. Chemical Engineering Journal, 86(1–2):17–23. https://doi.org/10.1016/S1385-8947(01)00267-4
Takeda M, Ueda A, Hashimot OH, et al., 2006. Fate of the chlorine and fluorine in a sub-bituminous coal during pyrolysis and gasification. Fuel, 85(2):235–242. https://doi.org/10.1016/j.fuel.2005.02.029
Xie HW, Zhang YF, Zhang Y, 2008. Numerical simulation and experimental study of flue gas cleaning in waste incineration power plants. Proceedings of the CSEE, 28(5): 17–22 (in Chinese).
Xu G, Yuan X, Yang YP, 2012. Optimization operation of flue gas desulfurization systems in power plants for energy conservation. Proceedings of the CSEE, 32(32):22–29 (in Chinese).
Xu X, Jiang XG, He J, et al., 2002. Experimental research on present form of chlorine in coal. Coal Geology and Exploration, 30(4):3–6 (in Chinese).
Zhang JB, Hao W, Zhao ZJ, et al., 2011. Theoretical and practical research on mechanism of low-temperature corrosion caused by boiler flue gas. Journal of Chinese Society of Power Engineering, 31(10):730–733 (in Chinese).
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the Zhejiang Provincial Natural Science Foundation of China (No. LY15E060002)
Rights and permissions
About this article
Cite this article
Liu, C., Zhao, H., Yang, Wy. et al. Chemical kinetics simulation of semi-dry dechlorination in coal-fired flue gas. J. Zhejiang Univ. Sci. A 19, 148–157 (2018). https://doi.org/10.1631/jzus.A1600653
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.A1600653