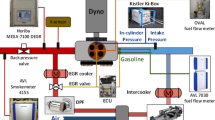
A reduced and optimized kinetic mechanism was built for coke oven gas (COG) as a clean alternative vehicle fuel. This mechanism was constructed by combining a reduced methane mechanism, an optimized H2/CO mechanism, and a reduced NOx formation mechanism based on the mechanism structure for simple hydrocarbon fuels. The key reactions for combustion were investigated by a sensitivity analysis model, and the kinetic parameters of these reactions were optimized within the uncertainty range by an optimization model based on particle swarm optimization (PSO). The ignition delay time and laminar flame speed were simulated using the optimized mechanism with the software of CHEMKIN, and the results agreed well with the relevant experimental data. A computational fluid dynamics (CFD) model coupled with the optimized mechanism was established using KIVA-CHEMKIN software, and the in-cylinder combustion process was simulated. The simulation results (in-cylinder pressure and NOx emission) showed good agreement with the engine bench test results.
Abstract
目的
化学反应机理在内燃机计算流体动力学(CFD)仿真中起关键作用。基于敏感性分析与粒子群 寻优算法,本文旨在提出适用于内燃机CFD仿真的焦炉气化学反应机理,为焦炉气在内燃机上的应用研究提供条件。
创新点
1. 结合敏感性分析与离子群寻优算法,对化学反应机理参数进行了优化;2. 建立了焦炉气 化学反应机理,可准确仿真滞燃期、层流火焰速度、缸内压力变化和NOx生成。
方法
1. 根据简单碳氢燃料机理结构,搭建焦炉气化学反应机理(图1);2. 通过敏感性分析,获得在燃烧中起关键作用的化 学反应(图2和3);3. 通过粒子群寻优算法,对上述关键化学反应的动力学参数进行优化(图4和5);4. 通过数 值仿真,验证机理的准确性(图6~13、16和17)
结论
1. 根据敏感性定义,搭建的敏感性分析模型可准确地识别在燃烧过程中起关键作用的化学反应;2. 基于粒子 群寻优算法搭建的优化模型可对化学反应的动力学参数进行合理优化;3. 优化后得到的焦炉气化学反应机理可准确预测滞燃 期与层流火焰速度以及模拟内燃机缸内压力变化与NOx 生成。
Similar content being viewed by others
References
Ahmed, S.S., Mauss, F., Moréac, G., et al., 2007. A comprehensive and compact n-heptane oxidation model derived using chemical lumping. Physical Chemistry Chemical Physics, 9(9):1107–1126. http://dx.doi.org/10.1039/B614712G
Baulch, D.L., Cobos, C.J., Cox, R.A., et al., 1994. Evaluated kinetic data for combustion modeling. Journal of Physical & Chemical Reference Data, 23(6):847–1033. http://dx.doi.org/10.1063/1.555953
Bhattacharjee, B., Schwer, D.A., Barton, P.I., et al., 2003. Optimally-reduced kinetic models: reaction elimination in large-scale kinetic mechanisms. Combustion & Flame, 135(3):191–208. http://dx.doi.org/10.1016/S0010-2180(03)00159-7
Bilger, R.W., Stårner, S.H., Kee, R.J., 1990. On reduced mechanisms for methane air combustion in nonpremixed flames. Combustion & Flame, 80(2):135–149. http://dx.doi.org/10.1016/0010-2180(90)90122-8
Boni, A.A., Penner, R.C., 1977. Sensitivity analysis of a mechanism for methane oxidation kinetics. Combustion Science & Technology, 15(3-4):99–106. http://dx.doi.org/10.1080/00102207708946775
Bougrine, S., Richard, S., Nicolle, A., et al., 2011. Numerical study of laminar flame properties of diluted methanehydrogen-air flames at high pressure and temperature using detailed chemistry. International Journal of Hydrogen Energy, 36(18):12035–12047. http://dx.doi.org/10.1016/j.ijhydene.2011.06.053
Bowman, C.T., 1992. Control of combustion-generated nitrogen oxide emissions: technology driven by regulation. Symposium (International) on Combustion, 24(1):859–878. http://dx.doi.org/10.1016/S0082-0784(06)80104-9
Davis, S.G., Law, C.K., 1998. Determination of and fuel structure effects on laminar flame speeds of C1 to C8 hydrocarbons. Combustion Science & Technology, 140(1-6):427–449. http://dx.doi.org/10.1080/00102209808915781
Davis, S.G., Joshi, A.V., Wang, H., et al., 2005. An optimized kinetic model of H2/CO combustion. Proceedings of the Combustion Institute, 30(1):1283–1292. http://dx.doi.org/10.1016/j.proci.2004.08.252
Dong, C., Zhou, Q., Zhao, Q., 2009. Experimental study on the laminar flame speed of hydrogen/carbon monoxide/air mixtures. Fuel, 88(10):1858–1863. http://dx.doi.org/10.1016/j.fuel.2009.04.024
Dong, C., Zhou, Q., Chen, X., et al., 2014. On the laminar flame speed of hydrogen, carbon monoxide, and natural gas mixtures with air: insights for a dual-fuel polygeneration system. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 36(4):393–401. http://dx.doi.org/10.1080/15567036.2010.538806
Dowdy, D.R., Smith, D.B., Taylor, S.C., 1991. The use of expanding spherical flames to determine burning velocities and stretch effects in hydrogen/air mixtures. Symposium (International) on Combustion, 23(1):325–332. http://dx.doi.org/10.1016/S0082-0784(06)80275-4
Frassoldati, A., Faravelli, T., Ranzi, E., 2007. The ignition, combustion and flame structure of carbon monoxide/ hydrogen mixtures. Note 1: detailed kinetic modeling of syngas combustion also in presence of nitrogen compounds. International Journal of Hydrogen Energy, 32(15):3471–3485. http://dx.doi.org/10.1016/j.ijhydene.2007.01.011
Gaydon, A.G., Hurle, I.R., 1963. The Shock Tube in Hightemperature Chemical Physics. Chapman & Hall, UK.
Gersen, S., Darmeveil, H., Levinsky, H., 2012. The effects of CO addition on the autoignition of H2, CH4 and CH4/H2 fuels at high pressure in an RCM. Combustion & Flame, 159(12):3472–3475. http://dx.doi.org/10.1016/j.combustflame.2012.06.021
Golovitchev, V., 2002. The Mechanism of Iso-octane. Chalmers University of Technology. http://www.tfd.chalmers.se/~valeri/MECH/
Gu, X.J., Haq, M.Z., Lawes, M., et al., 2000. Laminar burning velocity and Markstein lengths of methane–air mixtures. Combustion & Flame, 121(1-2):41–58. http://dx.doi.org/10.1016/S0010-2180(99)00142-X
Halter, F., Chauveau, C., Djebaili-Chaumeix, N., et al., 2005. Characterization of the effects of pressure and hydrogen concentration on laminar burning velocities of methane–hydrogen–air mixtures. Proceedings of the Combustion Institute, 30(1):201–208. http://dx.doi.org/10.1016/j.proci.2004.08.195
He, F., Li, Y.M., Wu, H.B., et al., 2013. A performance study of coke oven gas vehicle. Advanced Materials Research, 724-725:1201–1205. http://dx.doi.org/10.4028/www.scientific.net/AMR.724-725.1201
Huang, J., Hill, P.G., Bushe, W.K., 2004. Shock-tube study of methane ignition under engine-relevant conditions: experiments and modeling. Combustion & Flame, 136(1-2): 25–42. http://dx.doi.org/10.1016/j.combustflame.2003.09.002
Huang, Z., Zhang, Y., Zeng, K., et al., 2006. Measurements of laminar burning velocities for natural gas–hydrogen–air mixtures. Combustion & Flame, 146(1-2):302–311. http://dx.doi.org/10.1016/j.combustflame.2006.03.003
Hughes, K.J., Turányi, T., Clague, A.R., et al., 2001. Development and testing of a comprehensive chemical mechanism for the oxidation of methane. International Journal of Chemical Kinetics, 33(9):513–538. http://dx.doi.org/10.1002/kin.1048
Jazbec, M., Fletcher, D.F., Haynes, B.S., 2000. Simulation of the ignition of lean methane mixtures using CFD modelling and a reduced chemistry mechanism. Applied Mathematical Modelling, 24(8-9):689–696. http://dx.doi.org/10.1016/S0307-904X(00)00012-3
Kalitan, D.M., Mertens, J.D., Crofton, M.W., et al., 2007. Ignition and oxidation of lean CO/H2 fuel blends in air. Journal of Propulsion & Power, 23(6):1291–1301. http://dx.doi.org/10.2514/1.28123
Kee, R.J., Grcar, J.F., Smooke, M.D., 1985. PREMIX: a Fortran Program for Modeling Steady Laminar Onedimensional Premixed Flames. Technical Report No. SAND85-8249. Sandia National Laboratories, Livermore, USA.
Kee, R.J., Rupley, F.M., Meeks, E., et al., 1996. CHEMKINIII: a FORTRAN Chemical Kinetics Package for the Analysis of Gas-phase Chemical and Plasma Kinetics. Technical Report No. SAND96-8216.Sandia National Laboratories, Livermore, USA. http://dx.doi.org/10.2172/481621
Kennedy, J., Eberhart, R., 1995. Particle swarm optimization. Proceedings of IEEE International Conference on Neural Networks. http://dx.doi.org/10.1109/icnn.1995.488968
Konnov, A.A., 2000. Detailed Reaction Mechanism for Small Hydrocarbons Combustion. Release 0.5. http://homepages.vub.ac.be/akonnov
Krejci, M.C., Mathieu, O., Vissotski, A.J., et al., 2013. Laminar flame speed and ignition delay time data for the kinetic modeling of hydrogen and syngas fuel blends. Journal of Engineering for Gas Turbines & Power, 135(2):021503. http://dx.doi.org/10.1115/1.4007737
Kuo, K.K., 1986. Principles of Combustion. John Wiley & Sons, New York, USA.
Kwon, O.C., Faeth, G.M., 2001. Flame/stretch interactions of premixed hydrogen-fueled flames: measurements and predictions. Combustion and Flame, 124(4):590–610. http://dx.doi.org/10.1016/S0010-2180(00)00229-7
Li, J., Zhao, Z., Kazakov, A., et al., 2007. A comprehensive kinetic mechanism for CO, CH2O, and CH3OH combustion. International Journal of Chemical Kinetics, 39(3): 109–136. http://dx.doi.org/10.1002/kin.20218
Li, S.C., Williams, F.A., 2002. Reaction mechanisms for methane ignition. Journal of Engineering for Gas Turbines & Power, 124(3):471–480. http://dx.doi.org/10.1115/1.1377871
Liu, X., Liu, J.F., Zhao, W.B., et al., 2007. The particle swarm optimization algorithm restraining local optimum. Journal of Daqing Petroleum Institute, 31(6):101–104 (in Chinese). http://dx.doi.org/10.3969/j.issn.2095-4107.2007.06.029
Lu, T., Law, C.K., 2006. Linear time reduction of large kinetic mechanisms with directed relation graph: n-heptane and iso-octane. Combustion & Flame, 144(1-2):24–36. http://dx.doi.org/10.1016/j.combustflame.2005.02.015
Metghalchi, M.A.K.J., Keck, J.C., 1980. Laminar burning velocity of propane-air mixtures at high temperature and pressure. Combustion & Flame, 38:143–154. http://dx.doi.org/10.1016/0010-2180(80)90046-2
Michael, J.V., Su, M.C., Sutherland, J.W., et al., 2002. Rate constants for H+O2+M→HO2+M in seven bath gases. The Journal of Physical Chemistry A, 106(21):5297–5313. http://dx.doi.org/10.1021/jp020229w
Mittal, G., Sung, C.J., Yetter, R.A., 2006. Autoignition of H2/CO at elevated pressures in a rapid compression machine. International Journal of Chemical Kinetics, 38(8): 516–529. http://dx.doi.org/10.1002/kin.20180
Montgomery, C.J., Swensen, D.A., Harding, T.V., et al., 2002. A computational problem solving environment for creating and testing reduced chemical kinetic mechanisms. Advances in Engineering Software, 33(2):59–70. http://dx.doi.org/10.1016/S0965-9978(01)00054-0
Mueller, M.A., Yetter, R.A., Dryer, F.L., 1999. Flow reactor studies and kinetic modeling of the H2/O2/NOx, and CO/H2O/O2/NOx reactions. International Journal of Chemical Kinetics, 31(10):705–724. http://dx.doi.org/10.1002/(SICI)1097-4601(1999)31:10< 705::AID-JCK4>3.3.CO;2-R
Olm, C., Zsély, I.G., Varga, T., et al., 2015. Comparison of the performance of several recent syngas combustion mechanisms. Combustion & Flame, 162(5):1793–1812. http://dx.doi.org/10.1016/j.combustflame.2014.12.001
Petersen, E.L., Davidson, D.F., Hanson, R.K., 1999. Kinetics modeling of shock-induced ignition in low-dilution CH4/O2 mixtures at high pressures and intermediate temperatures. Combustion & Flame, 117(1-2):272–290. http://dx.doi.org/10.1016/S0010-2180(98)00111-4
Petersen, E.L., Kalitan, D.M., Barrett, A.B., et al., 2007. New syngas/air ignition data at lower temperature and elevated pressure and comparison to current kinetics models. Combustion & Flame, 149(1-2):244–247. http://dx.doi.org/10.1016/j.combustflame.2006.12.007
Qin, W.J., Xie, M.Z., Jia, M., et al., 2014. Large eddy simulation and proper orthogonal decomposition analysis of turbulent flows in a direct injection spark ignition engine: cyclic variation and effect of valve lift. Science China Technological Sciences, 57(3):489–504. http://dx.doi.org/10.1007/s11431-014-5472-x
Ra, Y., Reitz, R.D., 2008. A reduced chemical kinetic model for IC engine combustion simulations with primary reference fuels. Combustion & Flame, 155(4):713–738. http://dx.doi.org/10.1016/j.combustflame.2008.05.002
Rabitz, H., Kramer, M., Dacol, D., 1983. Sensitivity analysis in chemical kinetics. Annual Review of Physical Chemistry, 34:419–461. http://dx.doi.org/10.1146/annurev.pc.34.100183.002223
Ruscic, B., Feller, D., Dixon, D.A., et al., 2001. Evidence for a lower enthalpy of formation of hydroxyl radical and a lower gas-phase bond dissociation energy of water. ChemInform, 32(15):1–4. http://dx.doi.org/10.1002/chin.200115014
Saxena, P., Williams, F.A., 2006. Testing a small detailed chemical-kinetic mechanism for the combustion of hydrogen and carbon monoxide. Combustion & Flame, 145(1-2):316–323. http://dx.doi.org/10.1016/j.combustflame.2005.10.004
Scholte, T.G., Vaags, P.B., 1959. Burning velocities of mixtures of hydrogen, carbon monoxide and methane with air. Combustion & Flame, 3:511–524. http://dx.doi.org/10.1016/0010-2180(59)90057-4
Sher, E., Refael, S., 1988. A simplified reaction scheme for the combustion of hydrogen enriched methane/air flame. Combustion Science & Technology, 59(4-6):371–389. http://dx.doi.org/10.1080/00102208808947106
Shioji, M., Eguchi, S., Kitazaki, M., et al., 2004. Knock characteristics and performance in an SI engine with hydrogen and natural-gas blended fuels. SAE Technical Paper 2004-01-1929. http://dx.doi.org/10.4271/2004-01-1929
Smith, G.P., Golden, D.M., Frenklach, M., et al., 1999. GRI-Mech 3.0. University of California, Berkeley, USA. http://combustion.berkeley.edu/gri-mech/version30/text30. html
Smooke, M.D., 1991. Reduced Kinetic Mechanisms and Asymptotic Approximations for Methane-Air Flames: a Topical Volume. Springer Berlin Heidelberg. http://dx.doi.org/10.1007/BFb0035362
Sun, H., Yang, S.I., Jomaas, G., et al., 2007. High-pressure laminar flame speeds and kinetic modeling of carbon monoxide/hydrogen combustion. Proceedings of the Combustion Institute, 31(1):439–446. http://dx.doi.org/10.1016/j.proci.2006.07.193
Tan, Z., Reitz, R.D., 2006. An ignition and combustion model based on the level-set method for spark ignition engine multidimensional modeling. Combustion & Flame, 145(1-2):1–15. http://dx.doi.org/10.1016/j.combustflame.2005.12.007
Turányi, T., Nagy, T., Zsély, I.G., et al., 2012. Determination of rate parameters based on both direct and indirect measurements. International Journal of Chemical Kinetics, 44(5):284–302. http://dx.doi.org/10.1002/kin.20717
Turns, S.R., 1996. An Introduction to Combustion: Concepts and Applications. McGraw-Hill, USA. http://dx.doi.org/10.1036/007235044X
Varga, L., Szabó, B., Zsély, I.G., et al., 2011. Numerical investigation of the uncertainty of Arrhenius parameters. Journal of Mathematical Chemistry, 49(8):1798–1809. http://dx.doi.org/10.1007/s10910-011-9859-7
Wang, H., Frenklach, M., 1991. Detailed reduction of reaction mechanisms for flame modeling. Combustion & Flame, 87(3-4):365–370. http://dx.doi.org/10.1016/0010-2180(91)90120-Z
Warnatz, J., 2000. Hydrocarbon oxidation high-temperature chemistry. Pure & Applied Chemistry, 72(11):2101–2110. http://dx.doi.org/10.1351/pac200072112101
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the Major Project of Guizhou Province (No. 2012(6001)), China
ORCID: Hai-bin HE, http://orcid.org/0000-0002-1225-0272; Dong-wei YAO, http://orcid.org/0000-0001-7698-514X
Rights and permissions
About this article
Cite this article
He, HB., Yao, DW. & Wu, F. A reduced and optimized kinetic mechanism for coke oven gas as a clean alternative vehicle fuel. J. Zhejiang Univ. Sci. A 18, 511–530 (2017). https://doi.org/10.1631/jzus.A1600636
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.A1600636
Keywords
- Coke oven gas (COG)
- Kinetic mechanism
- Sensitivity analysis
- Particle swarm optimization (PSO)
- Spark-ignition (SI) engine
- Computational fluid dynamics (CFD) simulation