Abstract
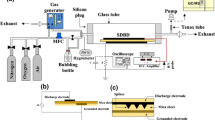
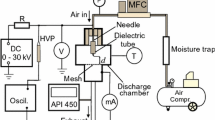
The naphthalene decomposition in a corona radical shower discharge (CRS) was investigated, with attention paid to the influences of voltage and initial naphthalene density. The OH emission spectra were investigated so as to know the naphthalene decomposing process. The by-products were analyzed and a decomposing theory in discharge was proposed. The results showed that higher voltage and relative humidity were effective on decomposition. The initial concentration affected the decomposing efficiency of naphthalene. When the initial naphthalene density was 17 mg/m3, the decomposition rate was found to be 70% under 14 kV. The main by-products were carbon dioxide and water. However, a small amount of carbonic oxide, 1,2-ethanediol and acetaldehyde were found due to the incomplete oxidization.
Similar content being viewed by others
References
Aranda, A., Lopez, J.M., Murillo, R., Mastral, A.M., Dejoz, A., Vazquez, I., Solsona, B., Yaylor, S.H., García, T., 2009. Total oxidation of naphthalene with high selectivity using a ceria catalyst prepared by a combustion method employing ethylene glycol. Journal of Hazardous Materials, 171(1–3):393–399. [doi:10.1016/j.jhazmat.2009.06.013]
Chang, J.S., Urashima, K., Tong, Y.X., Liu, W.P., Wei, H.Y., Yang, F.M., Liu, X.J., 2003. Simultaneous removal of NOx and SO2 from coal boiler flue gases by DC corona discharge ammonia radical shower system: pilot plant tests. Journal of Electrostatics, 57(3–4):313–323. [doi:10. 1016/S0304-3886(02)00168-7]
de Izarra, C., 2000. UV OH spectrum used as molecular pyrometer. Journal of Physics D: Applied Physics, 33(14): 1697–1704.
Dixon, J.K., Longfield, J.E., 1960. Hydrocarbon oxidation. Catalysis, 7:183–280.
Ershov, A., Borysow, J., 1995. Dynamics of OH (X2Π, ν=0) in high-energy atmospheric pressure electrical pulsed discharge. Journal of Physics D: Applied Physics, 28(1): 68–74. [doi:10.1088/0022-3727/28/1/012]
Falkenstein, Z., 1997. Influence of ultraviolet illumination on OH formation in dielectric barrier discharge of Ar/O2/H2O: the Joshi effect. Journal of Applied Physics, 81(11):7158–7162. [doi:10.1063/1.365313]
Gao, X., Shen, X., Wu, Z.L., Luo, Z.Y., Ni, M.J., Cen, K.F., 2009. The Mechanism of Naphthalene Decomposition in Corona Radical Shower System by DC Discharge. 11th International Conference on Electrostatic Precipitation, Hangzhou, China, p.713–717.
García, T., Solsona, B., Taylor, S.H., 2006. Naphthalene total oxidation over metal oxide catalysts. Applied Catalysis B: Environmental, 66(1–2):92–99. [doi:10.1016/j.apcatb.2006.03.003]
Gong, Z.Q., Alef, K., Wilke, B.M., Li, P.J., 2007. Activated carbon adsorption of PAHs from vegetable oil used in soil remediation. Journal of Hazardous Materials, 143(1–2): 372–378. [doi:10.1016/j.jhazmat.2006.09.037]
Hwang, G., Park, S.R., Lee, C.H., Ahn, I.S., Yoon, Y.J., Mhin, B.J., 2009. Influence of naphthalene biodegradation on the adhesion of Pseudomonas putida NCIB 9816-4 to a naphthalene-contaminated soil. Journal of Hazardous Materials, 172(1):491–493. [doi:10.1016/j.jhazmat.2009.07.009]
Lair, A., Ferronato, C., Chovelon, J.M., Herrmann, J.M., 2008. Naphthalene degradation in water by heterogeneous photocatalysis. Journal of Photochemistry and Photobiology A: Chemistry, 193(2-3):193–203. [doi:10.1016/j.jphotochem.2007.06.025]
Mastral, A.M., García, T., Callén, M.S., Navarro, M.V., Galbán, J., 2001. Removal of naphthalene phenanthrene, and pyrene by sorbents from hot gas. Environmental Science & Technology, 35(11):2395–2400. [doi:10.1021/es000152u]
Mista, W., Kacprzyk, R., 2008. Decomposition of toluene using non-thermal plasma reactor at room temperature. Catalysis Today, 137(2–4):345–349. [doi:10.1016/j.cattod.2008.02.009]
Nichipor, H., Dashouk, E., Yacko, S., Chmielewski, A.G., Zimek, Z., Sun, Y., 2002. Chlorinated hydrocarbons and PAH decomposition in dry and humid air by electron beam irradiation. Radiation Physics and Chemistry, 65(4–5):423–427. [doi:10.1016/S0969-806X(02)00352-3]
Nishino, N., Arey, J., Atkinson, R., 2009. Formation and reactions of 2-formylcinnamaldehyde in the OH radical-initiated reaction of naphthalene. Environmental Science & Technology, 43(5):1349–1353. [doi:10.1021/es802477s]
Onwudili, J.A., Williams, P.T., 2007. Reaction mechanisms for the decomposition of phenanthrene and naphthalene under hydrothermal conditions. The Journal of Supercritical Fluids, 39(3):399–408. [doi:10.1016/j.supflu.2006.03.014]
Pellerin, S., Cormier, J.M., Richard, F., Musiol, K., Chapelle, J., 1996. A spectroscopic diagnostic method using UV OH band spectrum. Journal of Physics D: Applied Physics, 29(3):726–739. [doi:10.1088/0022-3727/29/3/034]
Reed, D.R., Kass, S.R., 2000. Experimental determination of α and β C-H bond dissociation energies in naphthalene. Journal of Mass Spectrometry, 35(4):534–539. [doi:10.1002/(SICI)1096-9888(200004)35:4〈534::AID-JMS964〉3.0.CO;2-T]
Tamura, M., Berg, P.A., Harrington, J.E., Luque, J., Jeffries, J.B., Smith, G.P., Crosley, D.R., 1998. Collisional quenching of CH (A), OH (A) and NO (A) in low pressure hydrocarbon flames. Combustion and Flame, 114(3–4): 502–514. [doi:10.1016/S0010-2180(97)00324-6]
Urashima, K., Chang, J.S., 2000. Removal of volatile organic compounds from air streams and industrial flue gases by non-thermal plasma technology. IEEE Transactions on Dielectrics and Electrical Insulation, 7(5):602–614. [doi:10.1109/94.879356]
Vialaton, D., Richard, C., Baglio, D., 1999. Mechanism of the photochemical transformation of naphthalene in water. Journal of Photochemistry and Photobiology A: Chemistry, 123(1–3):15–19. [doi:10.1016/S1010-6030(99) 00044-1]
Wainwright, M.S., Foster, N.R., 1979. Catalysis, kinetics, and reactor design in phthalic anhydride synthesis. Catalysis Reviews, 19(2):211–292. [doi:10.1080/03602457908068056]
Wu, Z.L., Gao, X., Luo, Z.Y., Wei, E.Z., Zhang, Y.S., Zhang, J.Z., Ni, M.J., Cen, K.F., 2005. NOx treatment by DC corona radical shower with different geometric nozzle electrodes. Energy & Fuels, 19(6):2279–2286. [doi:10.1021/ef0400823]
Yu, L., Li, X.D., Tu, X., Wang, Y., Lu, S.Y., Yan, J.H., 2010. Decomposition of naphthalene by DC gliding arc gas discharge. The Journal of Physical Chemistry A, 114(1): 360–368. [doi:10.1021/jp905082s]
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Basic Research Program (973) of China (No. 2006CB200303), the Chinese-Slovak Scientific and Technological Cooperation Program (No. 2010DFA92020), and the China Postdoctoral Science Foundation (No. 20100471698)
Rights and permissions
About this article
Cite this article
Ni, Mj., Shen, X., Gao, X. et al. Naphthalene decomposition in a DC corona radical shower discharge. J. Zhejiang Univ. Sci. A 12, 71–77 (2011). https://doi.org/10.1631/jzus.A1010009
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.A1010009