Abstract
Objective
To conduct a randomized comparative trial of pharmacokinetics, efficacy and toxicity profile treatment with 1200 mg/m2 gemcitabine using standard 30-min infusion or fixed dose rate (FDR) infusion [10 mg/(m2·min)] on days 1 and 8 plus carboplatin AUC (area under curve) 5 on day 1 in Chinese non-small-cell cancer patients. Twelve patients were enrolled in this study.
Methods
Plasma gemcitabine concentrations were measured by ion-pair reversed phase high performance liquid chromatography. Antitumoral activity and toxicity of gemcitabine was assessed according to World Health Organization criteria.
Results
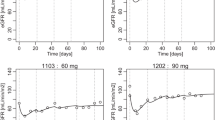
The obtained mean parameters, such as T 1/2 (elimination half time), AUC, and CL (clearance), were consistent with those reported in literature. Qualified response rate in our study was 33.3% for standard arm and 50% for FDR arm. Additional 50% and 33.3% patients contracted stable disease (SD) in standard arm and FDR arm, respectively. The predominant toxicity was hematologic, and patients in the standard infusion arm experienced consistently more hematologic toxicity.
Conclusion
Pharmacokinetic and clinical data in this trial support the continued evaluation of the FDR infusion strategy with gemcitabine.
Similar content being viewed by others
References
Abbruzzese, J.L., Grunewald, R., Weeks, E.A., Gravel, D., Adams, T., Nowak, B., Mineishi, S., Tarassoff, P., Satterlee, W., Raber, M.N., 1991. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J. Clin. Oncol., 9(3):491–498.
Abratt, R.P., Bezwoda, W.R., Falkson, G., Goedhal, L., Hacking, D., Rugg, T.A., 1994. Efficacy and safety profile of gemcitabine in non-small cell lung cancer: a phase II study. J. Clin. Oncol., 12(9):1535–1540.
Anderson, H., Lund, B., Bach, F., Thatcher, N., Walling, J., Hansen, H.H., 1994. Single-agent activity of weekly gemcitabine in advanced non-small cell lung cancer: a phase II study. J. Clin. Oncol., 12(9):1821–1826.
Bhargava, P., Marshall, J.L., Fried, K., Williams, M., Lefebvre, P., Dahut, W., Hanfelt, J., Gehan, E., Figuera, M., Hawkins, M.J., Rizvi, N.A., 2001. Phase I and pharmacokinetic study of two sequences of gemcitabine and docetaxel administered weekly to patients with advanced cancer. Cancer Chemother. Pharmacol., 48(2):95–103. [doi:10.1007/s002800100317]
Carrato, A., Garcia-Gomez, J., Alberola, V., 1999. Carboplatin (CARBO) in combination with gemcitabine (GEM) in advanced non-small cell lung cancer (NSCLC). Comparison of two consecutive phase II trials using different schedules. Proc. Am. Soc. Clin. Oncol., 18:abstract 1922.
Domine, M., Casado, V., Esteves, L., 2000. Phase II study of carboplatin (C)-gemcitabine (G) in advanced non-small cell lung cancer (NSCLC). Proc. Am. Soc. Clin. Oncol., 19:abstract 2107.
Green, M.R., 1996. Gemcitabine safety overview. Semin. Oncol., 23(5 Suppl. 10):32–35.
Grunewald, R., Kantarjian, H., Keating, M.J., Abbruzzese, J., Tarassoff, P., Plunkett, W., 1990. Pharmacologically directed design of the dose rate and schedule of 2′,2′-difluorodeoxycytidine (gemcitabine) administration in leukemia. Cancer Res., 50(21):6823–6826.
Guchelaar, H.J., Richel, D.J., van Knapen, A., 1996. Clinical, toxicological and pharmacological aspects of gemcitabine. Cancer Treat. Rev., 22(1):15–31. [doi:10.1016/S0305-7372(96)90014-6]
Kroep, J.R., Giaccone, G., Voorn, D.A., Smitt, E.F., Beijnen, J.H., Rosing, H., van Moorsel, C.J., van Groeningen, C.J., Postmus, P.E., Pinedo, H.M., Peters, G.J., 1999. Gemcitabine and paclitaxel: pharmacokinetic and pharmacodynamic interactions in patients with non-small-cell lung cancer. J. Clin. Oncol., 17(7):2190–2197.
Lund, B., Ryberg, M., Petersen, P.M., Anderson, H., Thatcher, N., Dombernowsky, P., 1994. Phase II study of gemcitabine (2′,2′-difluorodeoxycytidine) given as a twice weekly schedule to previously untreated patients with non-small cell lung cancer. Ann. Oncol., 5(9):852–853.
Manuel, D., Laura, G.E., Ana, L., 2002. A phase II trial of a two hour infusion of gemcitabine with carboplatin for advanced non small cell lung cancer (NSCLC). Ann. Oncol., 13(Suppl. 5):143–148.
Miller, A.B., Hoogstraten, B., Staquet, M., Winkler, A., 1981. Reporting results of cancer treatment. Cancer, 47(1): 207–214. [doi:10.1002/1097-0142(19810101)47:1〈207::AID-CNCR2820470134〉3.0.CO;2-6]
Sederholm, C., 2002. Gemcitabine (G) compared with gemcitabine plus carboplatin (GC) in advanced non-small cell lung cancer: a phase III study by the Swedish Lung Cancer Study Group (SLUSG). Proc. Am. Soc. Clin. Oncol., 21:abstract 1162.
Shord, S.S., Faucette, S.R., Gillenwater, H.H., Pescatore, S.L., Hawke, R.L., Socinski, M.A., Lindley, C., 2003. Gemcitabine pharmacokinetics and interaction with paclitaxel in patients with advanced non-small-cell lung cancer. Cancer Chemother. Pharmacol., 51(4):328–336.
Stani, S.C., Bajetta, E., de Candis, D., 2001. Front line chemotherapy with four different schedules of gemcitabine and carboplatin in stage IV non-small cell lung cancer (NSCLC). Eur. J. Cancer, 37(Suppl. 6):abstract 192.
Storniolo, A.M., Allerheiligen, S.R., Pearce, H.L., 1997. Preclinical, pharmacologic, and phase I studies of gemcitabine. Semin. Oncol., 24(2 Suppl. 7):2–7.
Tempero, M., Plunkett, W., Ruiz van Haperen, V., Hainsworth, J., Hochster, H., Lenzi, R., Abbruzzese, J., 2003. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J. Clin. Oncol., 21(18):3402–3408. [doi:10.1200/JCO.2003.09.140]
Yanagihara, R.H., Beck, M., 2000. Low dose carboplatin and gemcitabine in advanced non-small cell lung cancer: an active combination with low toxicity. Pro. Am. Sol. Clin. Oncol., 19:2072–2075.
Zatloukal, P., Petruzelka, L., 2002. Gemcitabine/carboplatin in advanced non-small-cell lung cancer. Lung Cancer, 38(Suppl. 2):33–36. [doi:10.1016/S0169-5002(02)00355-0]
Zatloukal, P., Petruzelka, L., Zemanova, M., 2001. Gemcitabine plus cisplatin (GCis) versus gemcitabine plus carboplatin (GCarb) in patients (pts) with non-small cell lung cancer (NSCLC) stage IIIb and IV: an interim analysis of a randomized trial. Proc. Am. Soc. Clin. Oncol., 20:abstract 1343.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project (No. 2004A028) supported by the Medical Science Research Foundation of Zhejiang Province, China
Rights and permissions
About this article
Cite this article
Wang, Lr., Liu, J., Huang, Mz. et al. Comparison of pharmacokinetics, efficacy and toxicity profile of gemcitabine using two different administration regimens in Chinese patients with non-small-cell lung cancer. J. Zhejiang Univ. - Sci. B 8, 307–313 (2007). https://doi.org/10.1631/jzus.2007.B0307
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1631/jzus.2007.B0307