Abstract
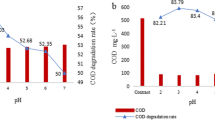
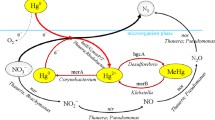
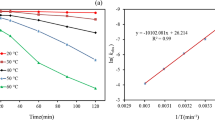
The aqueous phase oxidation of gaseous elemental mercury (Hg0) by potassium persulfate (KPS) catalyzed by Ag+ was investigated using a glass bubble column reactor. Concentration of gaseous mercury and potassium persulfate were measured by cold vapor atom absorption (CVAA) and ion chromatograph (IC), respectively. The effects of pH value, concentration of potassium persulfate and silver nitrate (SN), temperature, Hg0 concentration in the reactor inlet and tertiary butanol (TBA), free radical scavenger, on the removal efficiency of Hg0 were studied. The results showed that the removal efficiency of Hg0 increased with increasing concentration of potassium persulfate and silver nitrate, while temperature and TBA were negatively effective. Furthermore, the removal efficiency of Hg0 was much better in neutral solution than in both acidic and alkaline solution. But the influence of pH was almost eliminated by adding AgNO3. High Hg0 concentration has positive effect. The possible reaction mechanism of gaseous mercury was also discussed.
Similar content being viewed by others
References
Anipsitakis, G.P., Dionysiou, D.D., 2004. Transition metal/UV-based advanced oxidation technologies for water decontamination. Applied Catalysis B: Environmental, 54(3):155–163. [doi:10.1016/j.apcatb.2004.05.025]
Berlin, A.A., 1986. Kinetics of radical-chain decomposition of persulfate in aqueous solutions of organic compounds. Kinet. Catal., 27(1):34–39.
Dogliotti, L., Hayon, E., 1967. Flash photolysis of persulfate ions in aqueous solutions. Study of the sulfate and ozonide radical anions. J. Phys. Chem., 71(8):2511–2516. [doi:10.1021/j100867a019]
Hayon, E., McGarvey, J.J., 1967. Flash photolysis in the vacuum ultraviolet region of SO2− 4, CO2− 3 and OH− ions in aqueous solutions. J. Phys. Chem., 71(5):1472–1477. [doi:10.1021/j100864a044]
House, D.A., 1962. Kinetics and mechanism of oxidations by peroxydisulfate. Chem. Rev., 62(3):185–203. [doi:10.1021/cr60217a001]
Ivanov, K.L., Glebov, E.M., Plyusnin, V.F., Ivanov, Y.V., Grivin, V.P., Bazhin, N.M., 2000. Laser flash photolysis of sodium persulfate in aqueous solution with additions of dimethylformamide. Journal of Photochemistry and Photobiology A: Chemistry, 133(1–2):99–104. [doi:10.1016/S1010-6030(00)00218-5]
Kislenko, V.N., Berlin, A.A., Litovchenko, N.V., 1997. Kinetics of oxidation of glucose by persulfate ions in the presence of Mn(II) ions. Kinet. Catal., 38(3):391–396.
Kolthoff, I.M., Miller, I.K., 1951. The chemistry of persulfate: I. The kinetics and mechanism of the decomposition of the persulfate ion in aqueous medium. J. Am. Chem. Soc., 73(7):3055–3059. [doi:10.1021/ja01151a024]
Langlais, B., Reckhow, D.A., Brink, D.R., 1991. Ozone in Water Treatment—Applications and Engineering. Lewis Publishers, Chelsea, p.16–19.
Lenka, S., Dash, S.B., 1983. Polymerization of acrylonitrile initiated by potassium persulfate-cobalt(II) and potassium persulfate-manganese(II) redox system. J. Macromol. Sci. Chem. A, 20(3):397–407.
Liang, C.J., Bruell, C.J., Marley, M.C., Sperry, K.L., 2004a. Persulfate oxidation for in situ remediation of TCE. I. Catalyzed by ferrous ion with and without a persulfate-thiosulfate redox couple. Chemosphere, 55(9):1213–1223. [doi:10.1016/j.chemosphere.2004.01.029]
Liang, C.J., Bruell, C.J., Marley, M.C., Sperry, K.L., 2004b. Persulfate oxidation for in situ remediation of TCE. II. Catalyzed by chelated ferrous ion. Chemosphere, 55(9):1225–1233. [doi:10.1016/j.chemosphere.2004.01.030]
Lipfert, F.W., Moskowitz, P.D., Ftherakis, V., Dephillips, M., Viren, J., Saroff, L., 1995. Assessment of adult risks of paresthesia due to mercury from coal combustion. Water, Air and Soil Pollution, 80(1–4):1139–1148. [doi:10.1007/BF01189776]
Morita, H., Mitsuhashi, T., Sakurai, H., Shimomura, S., 1983. Absorption of mercury by solutions containing oxidants. Analytica Chimica Acta, 153(1):351–355. [doi:10.1016/S0003-2670(00)85528-2]
Nosov, E.F., 1966. Rate constant determination in the decomposition of potassium and ammonium peroxydisulfate in aqueous solution. Russ. J. Phys. Chem., 40:1571–1572.
Price, G.J., Clifton, A.A., 1996. Sonochemical acceleration of persulfate decomposition. Polymer, 37(17):3971–3973. [doi:10.1016/0032-3861(96)00197-8]
Skarzewski, J., 1984. Cerium catalyzed persulfate oxidation of polycyclic aromatic hydrocarbons to quinines. Tetrahedron, 40(23):4997–5000. [doi:10.1016/S0040-4020(01)91339-0]
Tanner, D.D., Osman, S.A.A., 1987. Oxidative decarbonation on the mechanism of potassium persulfate promoted decarbonation reaction. J. Org. Chem., 52(21):4689–4693. [doi:10.1021/jo00230a007]
US EPA, 1997. Mercury Study Report to Congress EPA-452/R-97-003. US EPA Office of Air Quality Planning and Standards, US Government Printing Office, Washington, DC.
US EPA, 1998. A Study of Hazardous Air Pollutant Emissions from Electric Utility Steam Generating Units: Final Report to Congress, EPA-453/R-98-004a. US EPA Office of Air Quality Planning and Standards, US Government Printing Office, Washington, DC.
van der Vaart, R., Akkerhuis, J., Feron, P., Jansen, B., 2001. Removal of mercury from gas streams by oxidative membrane gas absorption. Journal of Membrane Science, 187(1–2):151–157. [doi:10.1016/S0376-7388(01)00339-8]
Zhao, L.L., Rochelle, G.T., 1996. Hg absorption in aqueous permanganate. AIChE J., 42(12):3559–3562. [doi:10.1002/aic.690421227]
Zhao, L.L., Rochelle, G.T., 1998. Mercury absorption in aqueous oxidants catalyzed by mercury(II). Ind. Eng. Chem. Res., 37(2):380–387. [doi:10.1021/ie970155o]
Zhao, L.L., Rochelle, G.T., 1999. Mercury absorption in aqueous hypochlorite. Chem. Eng. Sci., 54(5):655–662. [doi:10.1016/S0009-2509(98)00263-2]
Author information
Authors and Affiliations
Additional information
Project (No. 20476094) supported by the National Natural Science Foundation of China
Rights and permissions
About this article
Cite this article
Ye, Qf., Wang, Cy., Wang, Dh. et al. Hg0 absorption in potassium persulfate solution. J. Zhejiang Univ. - Sci. B 7, 404–410 (2006). https://doi.org/10.1631/jzus.2006.B0404
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1631/jzus.2006.B0404