Abstract
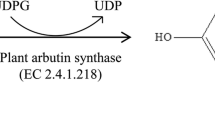
Arbutin was synthesized from glucose by two-step reaction below: (a) 2,3,4,6-tetra-O-acetyl-α-D-glucosyl chloride or bromide was prepared by glucose and acetyl halide (chloride or bromide). (b) 2,3,4,6-tetra-O-acetyl-α-D-glucosyl halide (Cl, Br) reacted with hydroquinone, methanol as solvent at pH=9.5∼10.0.
Similar content being viewed by others
References
Bárczai-Martos, M., Kórösy, F., 1950. Preparation of Acetobrome-Sugars. Nature, 165:369.
Chittenden, G.J.F., 1993. Reaction of some 1,2-trans-aldose peracetates with thionyl chloride-acetic acid-a convenient synthesis of some 1,2-trans-per-O-acetyl-D-glycosyl chlorides. Carbonhydr. Res., 242:297–301.
Cicchillo, R.M., Norris, P., 2000. A convenient synthesis of glycosyl chlorides from sugar hemiacetals using triphosgene as the chlorine source. Carbonhydr. Res., 328(3):431–434.
Ernst, B., Winkler, T., 1989. Preparation of glycosyl halides under neutral conditions. Tetrahedron Lett., 30(23):3081–3084.
Hwang, C.K., Li, W.S., Nicolaou, K.C., 1984. Reactions of hydroxyl groups with tosylchloride-dimethylaminopyridine system. Direct synthesis of chlorides from hydroxycompounds. Tetrahedron Lett., 25(22):2295–2296.
Karjala, S., Link, K.P., 1940. Synthesis of glycol glucosides. J. Am. Chem. Soc., 62(4):917–920.
Kartha, K.P.R., Jennings, H., 1990. A facile, one-step procedure for the conversion of 2-(trimethylsilyl) ethyl glycosides to their glycosyl chlorides. Tetrahedron Lett., 31(18):2537–2540.
Kovac, P., Edgar, K.J., 1992. Synthesis of ligands related to the O-specific antigen of type 1 Shigella dysenteriae. 3. Glycosylation of 4,6-O-substituted derivatives of methyl 2-acetamido-2-deoxy-.alpha.-D-glucopyranoside with glycosyl donors derived from mono- and oligosaccharides. J. Org. Chem., 57(8):2455–2467.
Kovac, P., Taylor, R.B., Glaudemans, C.P.J., 1985. General synthesis of (1. fwdarw. 3)-.beta.-D-galacto oligosaccharides and their methyl.beta.-glycosides by a stepwise or a blockwise approach. J. Org. Chem., 50(25):5323–5333.
Lemeux, R.U., 1963. Methods in Carbohydrate Chemistry. Academic Press, New York, U.S.A., p. 221.
Mukherjee, D., Ray, P.K., Chowdhury, U.S., 2001. Synthesis of glycosides via indium(III) chloride mediated activation of glycosyl halide in neutral condition. Tetrahedron, 57(36):7701–7704.
Ohrui, H., Fox, J.J., 1973. Nucleosides LXXXI. An approach to the synthesis of C-C linked β-D-ribofuranosyl nucleosides from 2,3-O-isopropylidene-5-O-trityl-β-D-ribofuranosyl chloride. Tetrahedron Lett., 14(22):1951–1954.
Pacsu, E., 1928. Über die Einwirkung von Titan (IV)-chlorid auf Zucker-Derivate, I.: Neue Methode zur Darstellung der Aceto-chlor-zucker und Umlagerung des β-Methyl-glucosidsinseine α-Form. Ber., 61:1508–1513.
Peromo, G.R., Krepinsky, J.J., 1987. A glycosylation reaction: Conversion of methyl glicosides to glycosyl chlorides by boron trichloride. Tetrahedron Lett., 28(46):5595–5598.
Pong, S.Q., Li, L.P., Wu, J.W., An, Y., Cheng, T.M., Cai, M.S., 1989. Synthesis of Arbutin Analogs. Acta Chimica Sinica, 47:512–515.
Author information
Authors and Affiliations
Additional information
Project supported by the National Natural Science Foundation of China (No. 20375036) and the Natural Science Foundation of Zhejiang Province (No. RC 0042), China
Rights and permissions
About this article
Cite this article
Huang, Sl., Zhu, Yl., Pan, Yj. et al. Synthesis of arbutin by two-step reaction from glucose. J. Zheijang Univ.-Sci. 5, 1509–1511 (2004). https://doi.org/10.1631/jzus.2004.1509
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.2004.1509
Key words
- Arbutin
- Synthesis
- 2,3,4,6-tetra-O-acetyl-α-D-glucosyl halide (chloride or bromide)
- Acetyl halide (Cl, Br)