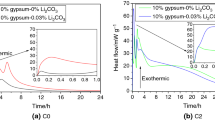
Abstract
Calcium sulfoaluminate-belite (CSAB) cement is considered a viable and environmentally friendly alternative to Portland cement. Ye'elimite or calcium sulfoaluminate which is the primary phase of CSAB cement undergoes rapid hydration, leading to the formation of ettringite or monosulfate as the major hydration product. The reactivity of ye'elimite can be influenced by other phases (e.g., calcium sulfate, calcium hydroxide, and alkalis) present in CSAB cement, affecting the evolution of hydrated phase assemblage. CSAB cements are known for their useful attributes such as quick setting, early-stage strength development, and the ability to compensate for shrinkage. These properties are directly linked to the early-age hydration of CSAB cement. This study is focused on the influence of alkali and calcium sulfate on the hydration of ye'elimite. Early-age hydration (up to 3 days) of laboratory-synthesized ye'elimite was studied under different alkali concentrations in the mix with or without calcium sulfate. The addition of gypsum was found to accelerate the early-age hydration of ye'elimite, and the alkalis contributed to further acceleration of the hydration in the initial hours. Alkalis were found to stabilize the hydration beyond a few hours, leading to a lower degree of hydration. Furthermore, alkalis influenced the formation as well as morphology of the hydration products, and the influence was more predominant in the absence of gypsum.












Similar content being viewed by others
Data availability
Data will be made available upon the request.
Notes
Cement chemistry notation: C = CaO, A = Al2O3, F = Fe2O3, S = SiO2, Ŝ = SO3.
References
Chen IA, Juenger MCG (2011) Synthesis and hydration of calcium sulfoaluminate-belite cements with varied phase compositions. J Mater Sci 46:2568–2577. https://doi.org/10.1007/s10853-010-5109-9
Winnefeld F, Lothenbach B (2010) Hydration of calcium sulfoaluminate cements—experimental findings and thermodynamic modelling. Cem Concr Res 40:1239–1247. https://doi.org/10.1016/j.cemconres.2009.08.014
Shenbagam VK, Cepuritis R, Chaunsali P (2021) Influence of exposure conditions on expansion characteristics of lime-rich calcium sulfoaluminate-belite blended cement. Cem Concr Compos 118:103932. https://doi.org/10.1016/j.cemconcomp.2021.103932
Guo X, Shi H, Hu W, Wu K (2014) Durability and microstructure of CSA cement-based materials from MSWI fly ash. Cem Concr Compos 46:26–31. https://doi.org/10.1016/j.cemconcomp.2013.10.015
El Khessaimi Y, El Hafiane Y, Smith A (2019) Examination of ye’elimite formation mechanisms. J Eur Ceram Soc 39:5086–5095. https://doi.org/10.1016/j.jeurceramsoc.2019.07.042
Li L, Wang R, Zhang S (2019) Effect of curing temperature and relative humidity on the hydrates and porosity of calcium sulfoaluminate cement. Construct Build Mater 213:627–636. https://doi.org/10.1016/j.conbuildmat.2019.04.044
Canbek O, Erdoğan ST (2020) Influence of production parameters on calcium sulfoaluminate cements. Constr Build Mater 239:117866. https://doi.org/10.1016/j.conbuildmat.2019.117866
Chaunsali P (2015) Early-age hydration and volume change of calcium sulfoaluminate cement-based binders. PhD Thesis. University of Illinois.
Miller SA, John VM, Pacca SA, Horvath A (2018) Carbon dioxide reduction potential in the global cement industry by 2050. Cem Concr Res 114:115–124. https://doi.org/10.1016/j.cemconres.2017.08.026
Chaunsali P, Vaishnav SK (2020) Calcium sulfoaluminate-belite cements: opportunities and challenges. Indian Concr J 94:18–25
Hanein T, Galvez-martos J, Bannerman MN (2018) Carbon footprint of calcium sulfoaluminate clinker production. J Clean Prod 172:2278–2287. https://doi.org/10.1016/j.jclepro.2017.11.183
Sharma A, Basavaraj AS, Chaunsali P, Gettu R (2023) Calcium sulfoaluminate cement manufacturing in india— prospects and prognosis of environmental impacts. ACI Mater J 120:17–28. https://doi.org/10.14359/51738456
Gartner E (2004) Industrially interesting approaches to “low-CO2” cements. Cem Concr Res 34:1489–1498. https://doi.org/10.1016/j.cemconres.2004.01.021
Kasselouri V, Tsakiridis P (1995) A study on the hydration products of a non-expansive sulfoaluminate cement. Cem Concr Res 25:1726–1736. https://doi.org/10.1016/0008-8846(95)00168-9
Jawed I, Skalny J (1978) Alkalies in cement: a review ii. effects of alkalies on hydration and performance of portland cement. Cem Concr Res 8:37-51. https://doi.org/10.1016/0008-8846(78)90056-X
Ribeiro DV, Labrincha JA, Morelli MR (2011) Potential use of natural red mud as pozzolan for Portland cement. Mater Res 14:60–66. https://doi.org/10.1590/S1516-14392011005000001
Canbek O, Shakouri S, Erdoğan ST (2020) Laboratory production of calcium sulfoaluminate cements with high industrial waste content. Cem Concr Compos 106:103475. https://doi.org/10.1016/j.cemconcomp.2019.103475
Chen IA (2009) Synthesis of Portland Cement and Calcium Sulfoaluminate-Belite Cement for Sustainable Development and Performance. PhD Thesis. The University of Texas at Austin.
Winnefeld F, Barlag S (2010) Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J Therm Anal Calorim 101:949–957. https://doi.org/10.1007/s10973-009-0582-6
Ogawa K, Roy DM (1982) C4A3S̄ hydration, ettringite formation, and its expansion mechanism: III. Effect of CaO, NaOH and NaCl; conclusions. Cem Concr Res 12:247–256. https://doi.org/10.1016/0008-8846(82)90011-4
Palou MT, Majling J (1996) Effects of sulphate, calcium and aluminum ions upon the hydration of sulphoaluminate belite cement. J Therm Anal 46:549–556. https://doi.org/10.1007/BF02135034
Zajac M, Skocek J, Bullerjahn F, Lothenbach B, Scrivener K, Ben Haha M (2019) Early hydration of ye’elimite: insights from thermodynamic modelling. Cem Concr Res 120:152–163. https://doi.org/10.1016/j.cemconres.2019.03.024
Tambara LUD, Cheriaf M, Rocha JC, Palomo A, Fernández-Jiménez A (2020) Effect of alkalis content on calcium sulfoaluminate (CSA) cement hydration. Cem Concr Res 128:105953. https://doi.org/10.1016/j.cemconres.2019.105953
Padilla-Encinas P, Palomo A, Blanco-Varela MT, Fernández-Jiménez A (2020) Calcium sulfoaluminate clinker hydration at different alkali concentrations. Cem Concr Res 138:106251. https://doi.org/10.1016/j.cemconres.2020.106251
Padilla-Encinas P, Fernández-Carrasco L, Palomo A, Fernández-Jiménez A (2022) Effect of alkalinity on early-age hydration in calcium sulfoaluminate clinker. Cem Concr Res 155:106781. https://doi.org/10.1016/j.cemconres.2022.106781
Zhang Y, Chang J (2018) Microstructural evolution of aluminum hydroxide gel during the hydration of calcium sulfoaluminate under different alkali concentrations. Constr Build Mater 180:655–664. https://doi.org/10.1016/j.conbuildmat.2018.06.010
Scrivener K, Snellings R, Lothenbach B (eds) (2016) A Practical Guide to Microstructural Analysis of Cementitious Materials. CRC Press (1st Edition). https://doi.org/10.1201/b19074
Hawthorne FC, Bladh KW, Burke EAJ, et al. (1987) New mineral names. Am Mineral 72:222–230. https://doi.org/10.2138/am-2018-NMN103234
Snellings R, Chwast J, Cizer Ö et al (2018) Report of TC 238-SCM: hydration stoppage methods for phase assemblage studies of blended cements—results of a round robin test. Mater Struct 51:111. https://doi.org/10.1617/s11527-018-1237-5
Taylor HFW, Famy C, Scrivener KL (2001) Delayed ettringite formation. Cem Concr Res 31:683–693. https://doi.org/10.1016/S0008-8846(01)00466-5
Ogawa K, Roy DM (1981) C4A3S hydration ettringite formation, and its expansion mechanism: I. expansion; Ettringite stability. Cem Concr Res 11:741–750. https://doi.org/10.1016/0008-8846(81)90032-6
Zhou Q, Glasser FP (2001) Thermal stability and decomposition mechanisms of ettringite at <120°C. Cem Concr Res 31:1333–1339. https://doi.org/10.1016/S0008-8846(01)00558-0
Fridrichová M, Dvořák K, Gazdič D, Mokrá J, Kulísek K (2016) Thermodynamic stability of ettringite formed by hydration of ye’elimite clinker. Adv Mater Sci Eng 2016:9280131. https://doi.org/10.1155/2016/9280131
Paul G, Boccaleri E, Buzzi L, Canonico F, Gastaldi D (2015) Friedel’s salt formation in sulfoaluminate cements: a combined XRD and 27Al MAS NMR study. Cem Concr Res 67:93–102. https://doi.org/10.1016/j.cemconres.2014.08.004
Gastaldi D, Paul G, Marchese L, Irico S, Boccaleri E, Mutke S, Buzzi L, Canonico F (2016) Hydration products in sulfoaluminate cements: evaluation of amorphous phases by XRD/solid-state NMR. Cem Concr Res 90:162–173. https://doi.org/10.1016/j.cemconres.2016.05.014
Hargis CW, Kirchheim AP, Monteiro PJM, Gartner EM (2013) Early age hydration of calcium sulfoaluminate (synthetic ye’elimite, C 4A3S̄) in the presence of gypsum and varying amounts of calcium hydroxide. Cem Concr Res 48:105–115. https://doi.org/10.1016/j.cemconres.2013.03.001
Sánchez-Herrero MJ, Fernández-Jiménez A, Palomo A (2013) C4A3Š hydration in different alkaline media. Cem Concr Res 46:41–49. https://doi.org/10.1016/j.cemconres.2013.01.008
Song F, Yu Z, Yang F, Lu Y, Liu Y (2015) Microstructure of amorphous aluminum hydroxide in belite-calcium sulfoaluminate cement. Cem Concr Res 71:1–6. https://doi.org/10.1016/j.cemconres.2015.01.013
Stark J, Bollmann K (2000) Delayed ettringite formation in concrete structures. Nord Concr Res 23:4–28.
Zhu Y, Liu Y, Zhang J (2022) Monitoring the hydration behavior of hardened cement paste affected by different environmental pH regimes. Front Mater 9. https://doi.org/10.3389/fmats.2022.980887
Gassó BM (2015) Impact of alkali salts on the kinetics and microstructural development of cementitious systems. PhD Thesis (No. 6763). EPFL.
Acknowledgements
The first author gratefully acknowledges the experimental facilities provided by the Department of Civil Engineering of IIT Madras and the Prime Minister's Research Fellowship from the Ministry of Education, India. The authors would like to acknowledge the Centre of Excellence on Technologies for Low-Carbon and Lean Construction at IIT Madras.
Author information
Authors and Affiliations
Contributions
Bipina Thaivalappil: Investigation, Methodology, Formal analysis, Writing-Original Draft. Vaishnav Kumar Shenbagam: Investigation, Methodology, Writing-Original Draft. Piyush Chaunsali: Conceptualization, Writing- Reviewing and Editing, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare no conflicts of interest related to the research, authorship, and/or publication of this article.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thaivalappil, B., Shenbagam, V.K. & Chaunsali, P. Early-age hydration of ye'elimite (calcium sulfoaluminate phase) in presence of alkalis: role of calcium sulfate. Mater Struct 57, 108 (2024). https://doi.org/10.1617/s11527-024-02384-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-024-02384-0