Abstract
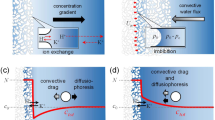
Understanding the diffusion behavior of solutions containing aggressive ions in saturated C–S–H nanopores is of significant importance. This simulation provides unprecedented microscopic images that reproduce the transport of solutions in saturated C–S–H channels based on molecular dynamics. Specifically, we show that the diffusion behavior of aggressive ions is hindered in saturated pore channels compared to unsaturated transport processes, leading to their reduced permeability. Due to the electrical double layers effect, The NaCl solution invasion in C–S–H nanopores is faster compared to the overflow of aqueous solutions. The hydration shells of anions and cations undergo different changes during the intrusion of ions into the C–S–H nanopores. For chloride ions with larger hydration shell radius, the adsorption capacity of the anion is significantly weakened with increasing pore size. While the hydration shell of sodium ions is more derived from the water molecules in the NaCl and is more easily hindered by the overflow of the aqueous solution in the saturated nanopore.















Similar content being viewed by others
References
Li MY, Ying YL, Yu J et al (2021) Revisiting the origin of nanopore current blockage for volume difference sensing at the atomic level. JACS Au 1(7):967–976
Payandeh, JS et al (2011) The crystal structure of a voltage-gated sodium channel. Nature 475(353).
Zhu Z, Wang D, Tian Y et al (2019) Ion/molecule transportation in nanopores and nanochannels: from critical principles to diverse functions. J Am Chem Soc 141(22):8658–8669
Shannon MA, Bohn PW, Elimelech M et al (2008) Science and technology for water purification in the coming decades. Nature, 452.
Zhang C, Lively RP, Zhang K et al (2012) Unexpected molecular sieving properties of zeolitic imidazolate framework-8. J Phys Chem Lett 3(16):2130–2134
Van Donk S, Janssen AH, Bitter JH et al (2003) Generation, characterization and impact of mesopores in zeolite catalysts. Catal Rev 45(2):297–319
Liu QL, et al (2015) A numerical study on chloride migration in cracked concrete using multi-component ionic transport models. Comput Mater Sci 99:396–416.
Mehta PK, Monteiro PJ (2013) Concrete: microstructure, properties, and materials. Prentice-Hall
Stambaugh ND, Bergman TL, Srubar WV (2018) Numerical service-life modeling of chloride-induced corrosion in recycled-aggregate concrete. Constr Build Mater 161:236–245
Aksoğan O, Binici H, Ortlek E (2016) Durability of concrete made by partial replacement of fine aggregate by colemanite and barite and cement by ashes of corn stalk, wheat straw and sunflower stalk ashes. Constr Build Mater 106:253–263
Bao J, Xue S, Zhang P et al (2021) Coupled effects of sustained compressive loading and freeze–thaw cycles on water penetration into concrete. Struct Concr 22:E944–E954
Cantero B, Bravo M, de Brito J et al (2021) Water transport and shrinkage in concrete made with ground recycled concrete-additioned cement and mixed recycled aggregate. Cem Concrete Compos, 118.
Rucker-Gramm P, Beddoe RE (2010) Effect of moisture content of concrete on water uptake. Cem Concr Res 40(1):102–108
Zhang P, Wang P, Hou D et al (2017) Application of neutron radiography in observing and quantifying the time-dependent moisture distributions in multi-cracked cement-based composites. Cement Concr Compos 78:13–20
Kuusela P, Pour-Ghaz M, Pini R et al (2021) Imaging of reactive transport in fractured cement-based materials with X-ray CT. Cem Concr Compos, 124.
Andrade C, Saucedo L, Rebolledo N et al (2020) X-Ray computed tomography and traditional analysis of a capillary absorption test in cement pastes. Cemt Concr Compos 113:103634
Voss A, Hosseini P, Pour-Ghaz M et al (2019) Three-dimensional electrical capacitance tomography–a tool for characterizing moisture transport properties of cement-based materials. Mater Des 181:107967
Smyl D, Hallaji M, Seppänen A et al (2016) Quantitative electrical imaging of three-dimensional moisture flow in cement-based materials. Int J Heat Mass Transf 103:1348–1358
Roels S, Carmeliet J, Hens H et al (2004) A comparison of different techniques to quantify moisture content profiles in porous building materials. J Therm Envelope Build Sci 27(4):261–276
Ping Y, Kirkpatrick RJ (2001) 35Cl NMR relaxation study of cement hydrate suspensions. Cem Concr Res 31(10):1479–1485
Yang J, Jia Y, Hou D et al (2019) Na and Cl immobilization by size controlled calcium silicate hydrate nanometer pores. Constr Build Mater 202:622–635
Youssef M, Pellenq RJ, Yildiz B (2011) Glassy nature of water in an ultraconfining disordered material: the case of calcium-silicate-hydrate. J Am Chem Soc 133(8):2499–2510
Hou D, Zhang W, Sun M et al (2020) Modified Lucas-Washburn function of capillary transport in the calcium silicate hydrate gel pore: a coarse-grained molecular dynamics study. Cem Concr Res 136:106166
Hou D, Li D, Yu J et al (2017) Insights on capillary adsorption of aqueous sodium chloride solution in the nanometer calcium silicate channel: a molecular dynamics study. J Phys Chem C 121(25):13786–13797
Li D, Zhao W, Hou D et al (2017) Molecular dynamics study on the chemical bound, physical adsorbed and ultra-confined water molecules in the nano-pore of calcium silicate hydrate. Constr Build Mater 151:563–574
Zhou Y, Hou D, Jiang J et al (2016) Chloride ions transport and adsorption in the nano-pores of silicate calcium hydrate: experimental and molecular dynamics studies. Constr Build Mater 126:991–1001
Hou D, Li Z (2014) Molecular dynamics study of water and ions transport in nano-pore of layered structure: a case study of tobermorite. Microporous Mesoporous Mater 195:9–20
Ma H, Li Z (2011) Multi-scale modeling of the microstructure of concrete. In: The Twenty-Fourth KKCNN Symposium on Civil Engineering
Yu J, Zheng Q, Hou D et al (2019) Insights on the capillary transport mechanism in the sustainable cement hydrate impregnated with graphene oxide and epoxy composite. Compos B Eng 173:106907
Yu J, Li SC, Hou DS et al (2019) Hydrophobic silane coating films for the inhibition of water ingress into the nanometer pore of calcium silicate hydrate gels. Phys Chem Chem Phys 21(35):19026–19038
Cygan RT, Liang J-J, Kalinichev AG (2004) Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J Phys Chem B 108(4):1255–1266
Mishra RK, Mohamed AK, Geissbühler D et al (2017) cemff: a force field database for cementitious materials including validations, applications and opportunities. Cem Concr Res 102:68–89
Liu Z, Wang Y, Xu D et al (2021) Multiple ions transport and interaction in calcium silicate hydrate gel nanopores: effects of saturation and tortuosity. Constr Build Mater, 283.
Plimpton S (1995) Fast parallel algorithms for short-range molecular dynamics. J Comput Phys 117(1):1–19
Hou D, Yu J, Wang P (2019) Molecular dynamics modeling of the structure, dynamics, energetics and mechanical properties of cement-polymer nanocomposite. Compos B Eng 162:433–444
Yang J, Hou D, Ding Q (2018) Ionic hydration structure, dynamics and adsorption mechanism of sulfate and sodium ions in the surface of calcium silicate hydrate gel: a molecular dynamics study. Appl Surf Sci 448:559–570
Hou D, Li T, Wang P (2018) Molecular dynamics study on the structure and dynamics of NaCl solution transport in the nanometer channel of CASH Gel. ACS Sustain Chem Eng 6(7):9498–9509
Fratini E, Faraone A, Ridi F et al (2013) Hydration water dynamics in tricalcium silicate pastes by time-resolved incoherent elastic neutron scattering. J Phys Chem C 117(14):7358–7364
Korb JP, Monteilhet L, McDonald PJ et al (2007) Microstructure and texture of hydrated cement-based materials: a proton field cycling relaxometry approach. Cem Concr Res 37(3):295–302
Acknowledgements
Financial support from the Natural Science Foundation of Shandong Province under Grant ZR2022QE225 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, J., Li, M. & Jin, Z. Exploration of saturated transport of ion concentration differences in C–S–H channels. Mater Struct 57, 8 (2024). https://doi.org/10.1617/s11527-023-02272-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-023-02272-z