Abstract
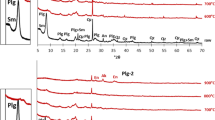
This article focuses on the use of two calcined marlstones as supplementary cementitious materials, one with palygorskite and smectite (MS1) as clay phases and the other with smectite only (MS2). The calcination and the reactivity of these two materials were first analysed by X-ray diffraction (XRD) and Magic Angle Spinning Solid State Nuclear Magnetic Resonance (MAS NMR). The two calcined marlstones were combined with Portland cement to produce mortars and measure compressive strength. The XRD and 27Al MAS NMR results showed that 800 °C is an optimal calcination temperature and that both calcined marlstones can be used as supplementary cementitious materials. The reactivity of MS1 was found to be higher than that of MS2. This was confirmed with compressive strength measurements which showed superior performance for mortars blended with calcined MS1 rather than calcined MS2. This difference between MS1 and MS2 is due to the presence of palygorskite in MS1, which greatly improves the reactivity and final mechanical performances. Therefore, palygorskite bearing marlstones are suitable for a use as SCM and this suggests that palygorskite exhibits a significant pozzolanic reactivity.







Similar content being viewed by others
References
Huntzinger DN, Eatmon TD (2009) A life-cycle assessment of Portland cement manufacturing: comparing the traditional process with alternative technologies. J Clean Prod 17:668–675. https://doi.org/10.1016/j.jclepro.2008.04.007
Scrivener KL, John VM, Gartner EM (2018) Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem Concr Res 114:2–26. https://doi.org/10.1016/j.cemconres.2018.03.015
Escalante JI, Gómez LY, Johal KK et al (2001) Reactivity of blast-furnace slag in Portland cement blends hydrated under different conditions. Cem Concr Res 31:1403–1409. https://doi.org/10.1016/S0008-8846(01)00587-7
Yazıcı H, Yardımcı MY, Yiğiter H et al (2010) Mechanical properties of reactive powder concrete containing high volumes of ground granulated blast furnace slag. Cement Concr Compos 32:639–648. https://doi.org/10.1016/j.cemconcomp.2010.07.005
Sakai E, Miyahara S, Ohsawa S et al (2005) Hydration of fly ash cement. Cem Concr Res 35:1135–1140. https://doi.org/10.1016/j.cemconres.2004.09.008
Yao ZT, Ji XS, Sarker PK et al (2015) A comprehensive review on the applications of coal fly ash. Earth Sci Rev 141:105–121. https://doi.org/10.1016/j.earscirev.2014.11.016
Hu X, Shi C, Shi Z, Zhang L (2019) Compressive strength, pore structure and chloride transport properties of alkali-activated slag/fly ash mortars. Cement Concr Compos 104:103392. https://doi.org/10.1016/j.cemconcomp.2019.103392
Alujas A, Fernández R, Quintana R et al (2015) Pozzolanic reactivity of low grade kaolinitic clays: Influence of calcination temperature and impact of calcination products on OPC hydration. Appl Clay Sci 108:94–101. https://doi.org/10.1016/j.clay.2015.01.028
Almenares RS, Vizcaíno LM, Damas S et al (2017) Industrial calcination of kaolinitic clays to make reactive pozzolans. Case Studies in Construction Materials 6:225–232. https://doi.org/10.1016/j.cscm.2017.03.005
El-Diadamony H, Amer AA, Sokkary TM, El-Hoseny S (2018) Hydration and characteristics of metakaolin pozzolanic cement pastes. HBRC Journal 14:150–158. https://doi.org/10.1016/j.hbrcj.2015.05.005
Zhao D, Khoshnazar R (2020) Microstructure of cement paste incorporating high volume of low-grade metakaolin. Cement Concr Compos 106:103453. https://doi.org/10.1016/j.cemconcomp.2019.103453
Brown IW, MacKenzie KJ, Meinhold RH (1987) The thermal reactions of montmorillonite studied by high-resolution solid-state29Si and27Al NMR. J Mater Sci 22(9):3265–3275
Garg N, Skibsted J (2014) Thermal activation of a pure montmorillonite clay and its reactivity in cementitious systems. J Phys Chem C 118:11464–11477. https://doi.org/10.1021/jp502529d
Kaminskas R, Kubiliute R, Prialgauskaite B (2020) Smectite clay waste as an additive for Portland cement. Cement Concr Compos 113:103710. https://doi.org/10.1016/j.cemconcomp.2020.103710
Fernandez R, Martirena F, Scrivener KL (2011) The origin of the pozzolanic activity of calcined clay minerals: a comparison between kaolinite, illite and montmorillonite. Cem Concr Res 41:113–122. https://doi.org/10.1016/j.cemconres.2010.09.013
Taylor-Lange SC, Rajabali F, Holsomback NA et al (2014) The effect of zinc oxide additions on the performance of calcined sodium montmorillonite and illite shale supplementary cementitious materials. Cement Concr Compos 53:127–135. https://doi.org/10.1016/j.cemconcomp.2014.06.008
Garg N, Skibsted J (2016) Pozzolanic reactivity of a calcined interstratified illite/smectite (70/30) clay. Cem Concr Res 79:101–111. https://doi.org/10.1016/j.cemconres.2015.08.006
Cancio Díaz Y, Sánchez Berriel S, Heierli U et al (2017) Limestone calcined clay cement as a low-carbon solution to meet expanding cement demand in emerging economies. Develop Eng 2:82–91. https://doi.org/10.1016/j.deveng.2017.06.001
Scrivener K, Martirena F, Bishnoi S, Maity S (2018) Calcined clay limestone cements (LC3). Cem Concr Res 114:49–56. https://doi.org/10.1016/j.cemconres.2017.08.017
He C, Makovicky E, Osbæck B (1996) Thermal treatment and pozzolanic activity of sepiolite. Appl Clay Sci 10:337–349. https://doi.org/10.1016/0169-1317(95)00035-6
He C, Osbaeck B, Makovicky E (1995) Pozzolanic reactions of six principal clay minerals: activation, reactivity assessments and technological effects. Cem Concr Res 25:1691–1702. https://doi.org/10.1016/0008-8846(95)00165-4
Justnes H, Østnor T, De Weerdt K, Vikan H (2011) Calcined marl and clay as mineral addition for more sustainable concrete structure. In Proceedings of the 36th Conference on Our World in Concrete & Structures, Singapore, 14–16
Danner T, Norden G, Justnes H (2018) Characterisation of calcined raw clays suitable as supplementary cementitious materials. Appl Clay Sci 162:391–402. https://doi.org/10.1016/j.clay.2018.06.030
Bullerjahn F, Zajac M, Pekarkova J, Nied D (2020) Novel SCM produced by the co-calcination of aluminosilicates with dolomite. Cem Concr Res 134:106083. https://doi.org/10.1016/j.cemconres.2020.106083
Mohammed S, Elhem G, Mekki B (2016) Valorization of pozzolanicity of Algerian clay: optimization of the heat treatment and mechanical characteristics of the involved cement mortars. Appl Clay Sci 132–133:711–721. https://doi.org/10.1016/j.clay.2016.08.027
Danner T, Norden G, Justnes H (2021) Calcareous smectite clay as a pozzolanic alternative to kaolin. Eur J Environ Civ Eng 25:1647–1664. https://doi.org/10.1080/19648189.2019.1590741
Bahhou A, Taha Y, Khessaimi YE et al (2021) Using calcined marls as non-common supplementary cementitious materials—a critical review. Minerals 11:517. https://doi.org/10.3390/min11050517
Poussardin V, Paris M, Wilson W, Tagnit-Hamou A, Deneele D (2022) Self-reactivity of a calcined palygorskite-bearing marlstone for potential use as supplementary cementitious material. Appl Clay Sci 216:106372
Poussardin V, Paris M, Tagnit-Hamou A, Deneele D (2020) Potential for calcination of a palygorskite-bearing argillaceous carbonate. Appl Clay Sci 198:105846. https://doi.org/10.1016/j.clay.2020.105846
Poussardin V, Paris M, Wilson W et al (2022) Self-reactivity of a calcined palygorskite-bearing marlstone for potential use as supplementary cementitious material. Appl Clay Sci 216:106372. https://doi.org/10.1016/j.clay.2021.106372
Ferraz E, Andrejkovicova S, Hajjaji W, Velosa AL, Silva AS, Rocha F (2015) Pozzolanic activity of metakaolins by the French standard of the modified Chapelle test: a direct methology. Acta Geodyn et Geomaterialia 12(3):289–298. https://doi.org/10.13168/AGG.2015.0026
Avet F, Snellings R, Alujas Diaz A et al (2016) Development of a new rapid, relevant and reliable (R3) test method to evaluate the pozzolanic reactivity of calcined kaolinitic clays. Cem Concr Res 85:1–11. https://doi.org/10.1016/j.cemconres.2016.02.015
Doebelin N, Kleeberg R (2015) Profex: a graphical user interface for the Rietveld refinement program BGMN. J Appl Crystallogr 48:1573–1580. https://doi.org/10.1107/S1600576715014685
Massiot D, Fayon F, Capron M et al (2002) Modelling one- and two-dimensional solid-state NMR spectra: modelling 1D and 2D solid-state NMR spectra. Magn Reson Chem 40:70–76. https://doi.org/10.1002/mrc.984
C01 Committee Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM International
Bala P, Samantaray BK, Srivastava SK (2000) Dehydration transformation in Ca-montmorillonite. Bull Mater Sci 23:61–67. https://doi.org/10.1007/BF02708614
Morodome S, Kawamura K (2009) Swelling behavior of Na- and Ca-Montmorillonite up to 150°C by in situ X-ray diffraction experiments. Clays Clay Miner 57:150–160. https://doi.org/10.1346/CCMN.2009.0570202
Xie J, Chen T, Xing B et al (2016) The thermochemical activity of dolomite occurred in dolomite–palygorskite. Appl Clay Sci 119:42–48. https://doi.org/10.1016/j.clay.2015.07.014
Maia AÁB, Angélica RS, de Freitas NR et al (2014) Use of 29Si and 27Al MAS NMR to study thermal activation of kaolinites from Brazilian Amazon kaolin wastes. Appl Clay Sci 87:189–196. https://doi.org/10.1016/j.clay.2013.10.028
Sanz J, Serratosa JM (1984) Silicon-29 and aluminum-27 high-resolution MAS-NMR spectra of phyllosilicates. J Am Chem Soc 106:4790–4793. https://doi.org/10.1021/ja00329a024
Müller D, Gessner W, Samoson A, Lippmaa E, Scheler G (1986) Solid-state aluminium-27 nuclear magnetic resonance chemical shift and quadrupole coupling data for condensed AlO 4 tetrahedra. J Chem Soc Dalton Trans 6:1277–1281
Lippmaa E, Maegi M, Samoson A et al (1980) Structural studies of silicates by solid-state high-resolution silicon-29 NMR. J Am Chem Soc 102:4889–4893. https://doi.org/10.1021/ja00535a008
Mackenzie KJD, Brown IWM, Cardile CM, Meinhold RH (1987) The thermal reactions of muscovite studied by high-resolution solid-state 29-Si and 27-Al NMR. J Mater Sci 22:2645–2654. https://doi.org/10.1007/BF01082158
Kuang W, Facey GA, Detellier C (2004) Dehydration and rehydration of palygorskite and the influence of water on the nanopores. Clays Clay Miner 52:635–642. https://doi.org/10.1346/CCMN.2004.0520509
Barron PF, Frost RL, Qlil N (1985) Solid state 29Si NMR examination of the 2:1 ribbon magnesium silicates, sepiolite and palygorskite. Am Miner 70:758–766
MacKenzie KJD, Smith ME (2002) Multinuclear solid-state NMR of Inorganic Materials. Elsiever, Berlin
Skibsted J, Jakobsen HJ, Hall C (1995) Quantification of calcium silicate phases in Portland cements by 29Si MAS NMR spectroscopy. J Chem Soc, Faraday Trans 91:4423. https://doi.org/10.1039/ft9959104423
Cherney EA, Hooton RD (1987) Cement growth failure mechanism in porcelain suspension insulators. IEEE Trans Power Delivery 2:249–255. https://doi.org/10.1109/TPWRD.1987.4308096
Magi M, Lippmaa E, Samoson A et al (1984) Solid-state high-resolution silicon-29 chemical shifts in silicates. J Phys Chem 88:1518–1522. https://doi.org/10.1021/j150652a015
Andersen MD, Jakobsen HJ, Skibsted J (2004) Characterization of white Portland cement hydration and the C-S-H structure in the presence of sodium aluminate by 27Al and 29Si MAS NMR spectroscopy. Cem Concr Res 34:857–868. https://doi.org/10.1016/j.cemconres.2003.10.009
Shoval S (1988) Mineralogical changes upon heating calcitic and dolomitic marl rocks. Thermochim Acta 135:243–252. https://doi.org/10.1016/0040-6031(88)87393-3
Hughes DC, Jaglin D, Kozłowski R, Mucha D (2009) Roman cements — Belite cements calcined at low temperature. Cem Concr Res 39:77–89. https://doi.org/10.1016/j.cemconres.2008.11.010
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Poussardin, V., Paris, M., Wilson, W. et al. Calcined palygorskite and smectite bearing marlstones as supplementary cementitious materials. Mater Struct 55, 224 (2022). https://doi.org/10.1617/s11527-022-02053-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-022-02053-0