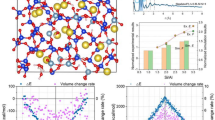
Abstract
Alkali-silica reaction (ASR) products exhibit viscoelastic behavior. Creep of nanolayered adsorbing materials is associated with shear deformations at the molecular scale. Crystalline ASR products are layered silicates with potassium and/or sodium as a charge balance ion in the interlayer space. These products are formed only in small quantities, exhibit long-range disorder, and are often mixed with other phases present in hydrated cement systems. In this context, experimental assessment of these products is a challenge and atomistic simulations arise as a valuable alternative. In this work, the shear response of crystalline ASR products is studied using classical molecular simulations. Understanding shear deformations are important to fully characterize the behavior of ASR products and may constitute a first step towards the comprehension of the viscoelastic behavior of these products. Further, the charge balance cation affects the shear modulus, von Mises yield stress, and residual strain, showing that the chemistry plays a crucial role in the mechanical response of these layered materials.






Similar content being viewed by others
References
Kirkpatrick RJ (2005) Experimental and molecular dynamics modeling studies of interlayer swelling: water incorporation in kanemite and ASR gel. Mater Struct 38(278):449
Shi Z, Geng G, Leemann A, Lothenbach B (2019) Synthesis, characterization, and water uptake property of alkali-silica reaction products. Cem Concr Res 121:58
Vollpracht A, Lothenbach B, Snellings R, Haufe J (2016) The pore solution of blended cements: a review. Mater Struct 49(8):3341
Honorio T, Benboudjema F, Bore T, Ferhat M, Vourc’h E (2019) The pore solution of cement-based materials: structure and dynamics of water and ions from molecular simulations. Phys Chem Chem Phys 21:11111
Benmore CJ, Monteiro PJM (2010) The structure of alkali silicate gel by total scattering methods. Cem Concr Res 40(6):892
Leemann A, Katayama T, Fernandes I, Broekmans MATM (2016) Types of alkali-aggregate reactions and the products formed. In: Proceedings of the Institution of Civil Engineers—Construction Materials 169(3):128
Dupuis R, Béland LK, Pellenq RJM (2019) Molecular simulation of silica gels: formation, dilution, and drying. Phys Rev Mater 3(7):075603
Carrier B, Vandamme M, Pellenq RJM, Bornert M, Ferrage E, Hubert F, Van Damme H (2016) Effect of water on elastic and creep properties of self-standing clay films. Langmuir 32(5):1370. https://doi.org/10.1021/acs.langmuir.5b03431
Morshedifard A, Masoumi S, Qomi MJA (2018) Nanoscale origins of creep in calcium silicate hydrates. Nat Commun 9(1):1785. https://doi.org/10.1038/s41467-018-04174-z
Zhang C, Sorelli L, Fournier B, Duchesne J, Bastien J, Chen Z (2017) Stress-relaxation of crystalline alkali-silica reaction products: characterization by micro- and nanoindentation and simplified modeling. Constr Build Mater 148:455
Sellier A, Multon S, Buffo-Lacarrière L, Vidal T, Bourbon X, Camps G (2016) Concrete creep modelling for structural applications: non-linearity, multi-axiality, hydration, temperature and drying effects. Cem Concr Res 79:301
Honorio T, Chemgne Tamouya OM, Shi Z, Bourdot A (2020) Intermolecular interactions of nanocrystalline alkali-silica reaction products under sorption. Cem Concr Res 136:106155. https://doi.org/10.1016/j.cemconres.2020.106155
Zubkova NV, Filinchuk YE, Pekov IV, Pushcharovsky DY, Gobechiya ER (2010) Crystal structures of shlykovite and cryptophyllite: comparative crystal chemistry of phyllosilicate minerals of the mountainite family. Eur J Mineral 22(4):547
Honorio T (2019) Monte Carlo molecular modeling of temperature and pressure effects on the interactions between crystalline calcium silicate hydrate layers. Langmuir 35(11):3907
Plimpton S (1995) Fast parallel algorithms for short-range molecular dynamics. J Comput Phys 117(1):1
Cygan RT, Liang JJ, Kalinichev AG (2004) Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J Phys Chem B 108(4):1255
Manzano H, Masoero E, Lopez-Arbeloa I, Jennings HM (2013) Shear deformations in calcium silicate hydrates. Soft Matter 9(30):7333. https://doi.org/10.1039/c3sm50442e
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52(12):7182. https://doi.org/10.1063/1.328693
Honorio T, Tamouya OMC, Shi Z (2020) Specific ion effects control the thermoelastic behavior of nanolayered materials: the case of crystalline alkali-silica reaction products. Phys Chem Chem Phys. https://doi.org/10.1039/D0CP04955G
Nedderman RM (2005) Statics and kinematics of granular materials. Cambridge University Press, Cambridge
Geng G, Shi Z, Leemann A, Glazyrin K, Kleppe A, Daisenberger D, Churakov S, Lothenbach B, Wieland E, Dähn R (2020) Mechanical behavior and phase change of alkali-silica reaction products under hydrostatic compression. Acta Crystallogr Sect B Struct Sci Cryst Eng Mater 76(4):674. https://doi.org/10.1107/S205252062000846X
Leemann A, Shi Z, Wyrzykowski M, Winnefeld F (2020) Moisture stability of crystalline alkali-silica reaction products formed in concrete exposed to natural environment. Mater Des 195:109066
Leemann A, Shi Z, Lindgård J (2020) Characterization of amorphous and crystalline ASR products formed in concrete aggregates. Cem Concr Res 137:106190
Bazant ZP, Hauggaard AB, Baweja S, Ulm FJ (1997) Microprestress-solidification theory for concrete creep. I: aging and drying effects. J Eng Mech 123(11):1188
Honorio T, Brochard L, Vandamme M, Lebée A (2018) Flexibility of nanolayers and stacks: implications in the nanostructuration of clays. Soft Matter 14(36):7354
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author declares that he have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Honorio, T. Shear deformations in crystalline alkali-silica reaction products at the molecular scale: anisotropy and role of specific ion effects. Mater Struct 54, 86 (2021). https://doi.org/10.1617/s11527-021-01671-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-021-01671-4