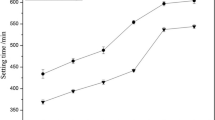
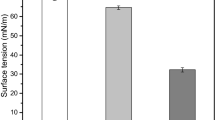
Abstract
In this work, shrinkage performance of ordinary portland cement (OPC) paste containing various alkali salts was characterized at two drying conditions, namely: nitrogen gas and air. The results show that incorporation of alkalis dramatically increases shrinkage magnitude, but reduces shrinkage kinetics of OPC, regardless of source and type of alkalis (e.g. Na+ or K+). The amount of alkalis bound in the solid hydrated phases, rather than the free alkalis remaining in the pore solution, is crucial in controlling the shrinkage performance of OPC. It is suggested that the alkali enrichment in OPC increases the visco-elastic/visco-plastic compliance (reduce creep modulus) of its solid skeleton under drying-induced internal stresses. This phenomenon is likely to be attributed to the alkalis binding in calcium–silicate–hydrate (C–S–H), which promotes the packing of C–S–H nanoparticles. Carbonation results in shrinkage (i.e. carbonation shrinkage) in plain OPC, but expansion in OPC with alkali enrichment. The overall volume change of OPC due to carbonation may be a result of competition between dissolution-induced shrinkage and crystallization-induced expansion.







Similar content being viewed by others
References
Juenger M, Winnefeld F, Provis J, Ideker J (2011) Advances in alternative cementitious binders. Cem Concr Res 41(12):1232–1243
Thomas RJ, Ye H, Radlińska A, Peethamparan S (2016) Alkali-activated slag concrete: a closer look at sustainable alternatives to portland cement. Concr Int 38(1):33–38
Sant G, Kumar A, Patapy C, Le Saout G, Scrivener K (2012) The influence of sodium and potassium hydroxide on volume changes in cementitious materials. Cem Concr Res 42(11):1447–1455
Beltzung F, Wittmann F, Holzer L (2001) Influence of composition of pore solution on drying shrinkage. In: Ulm F-J, Bazant ZP, Wittmann FH (eds) Creep, shrinkage and durability mechanics of concrete and other quasi-brittle materials. Elsevier Science Ltd, Amsterdam
Blaine RA (1968) Statistical study of the effects of trace elements on the properties of Portland cement. In: Proc. 5th Int’l symp. chemistry of cement. Tokyo, pp 86–91
Beltzung F, Wittmann F, Wan X, Sun W, van Breugel K, Miao C, Ye G, Chen H (2008) Influence of alkali content on creep and shrinkage of cement-based materials. In: International conference on microstructure related durability of cementitious composites. RILEM Publications, pp 905–915
Jawed I, Skalny J (1978) Alkalies in cement: a review: II. Effects of alkalies on hydration and performance of Portland cement. Cem Concr Res 8(1):37–51
Ye H, Radlińska A (2016) Quantitative analysis of phase assemblage and chemical shrinkage of alkali-activated slag. J Adv Concr Technol 14:245–260
Juenger MCG, Jennings HM (2001) Effects of high alkalinity on cement pastes. ACI Mater J 98(3):251–255
He Z, Li Z (2005) Influence of alkali on restrained shrinkage behavior of cement-based materials. Cem Concr Res 35(3):457–463
Maslehuddin M, Page C, Rasheeduzzafar (1996) Effect of temperature and salt contamination on carbonation of cements. J Mater Civ Eng 8(2):63–69
Kobayashi K, Uno Y (1990) Influence of alkali on carbonation of concrete, part 2-influence of alkali in cement on rate of carbonation of concrete. Cem Concr Res 20(4):619–622
Neville AM (1995) Properties of concrete, 4th ed. Pearson, Harlow
Verbeck GJ (1958) Carbonation of hydrated Portland cement. In: Committee C (ed) Cement and Concrete, STP39460S. ASTM International, West Conshohocken, pp 17–36. https://doi.org/10.1520/STP39460S
Powers TC (1900) A hypothesis on carbonation shrinkage, vol 4. Portland Cement Association
En Ruiz-Agudo, Kudłacz K, Putnis CV, Putnis A, Rodriguez-Navarro C (2013) Dissolution and carbonation of portlandite [Ca (OH) 2] single crystals. Environ Sci Technol 47(19):11342–11349
Chen JJ, Thomas JJ, Jennings HM (2006) Decalcification shrinkage of cement paste. Cem Concr Res 36(5):801–809
Matsushita F, Aono Y, Shibata S (2004) Calcium silicate structure and carbonation shrinkage of a tobermorite-based material. Cem Concr Res 34(7):1251–1257
Lodeiro IG, Macphee D, Palomo A, Fernández-Jiménez A (2009) Effect of alkalis on fresh C–S–H gels. FTIR analysis. Cem Concr Res 39(3):147–153
Mendoza O, Giraldo C, Camargo SS, Tobón JI (2015) Structural and nano-mechanical properties of calcium silicate hydrate (CSH) formed from alite hydration in the presence of sodium and potassium hydroxide. Cem Concr Res 74:88–94
Mota B, Matschei T, Scrivener K (2015) The influence of sodium salts and gypsum on alite hydration. Cem Concr Res 75:53–65
Ye H, Radlińska A (2016) Shrinkage mechanisms of alkali-activated slag. Cem Concr Res 88:126–135
Ye H, Cartwright C, Rajabipour F, Radlińska A (2017) Understanding the drying shrinkage performance of alkali-activated slag mortars. Cement Concr Compos 76:13–24
Ye H, Cartwright C, Rajabipour F, Radlińska A (2014) Effect of drying rate on shrinkage of alkali-activated slag cements. In: 4th international conference on the durability of concrete structure (ICDCS). Purdue University, Indiana, pp 254–261
Barneyback R, Diamond S (1981) Expression and analysis of pore fluids from hardened cement pastes and mortars. Cem Concr Res 11(2):279–285
Glasser F, Kindness A, Stronach S (1999) Stability and solubility relationships in AFm phases: part I. Chloride, sulfate and hydroxide. Cem Concr Res 29(6):861–866
Kumar A, Sant G, Patapy C, Gianocca C, Scrivener KL (2012) The influence of sodium and potassium hydroxide on alite hydration: experiments and simulations. Cem Concr Res 42(11):1513–1523
Viallis H, Faucon P, Petit J-C, Nonat A (1999) Interaction between salts (NaCl, CsCl) and calcium silicate hydrates (CSH). J Phys Chem B 103(25):5212–5219
Lothenbach B, Nonat A (2015) Calcium silicate hydrates: solid and liquid phase composition. Cem Concr Res 78:57–70
Ye H, Radlińska A (2016) A review and comparative study of existing shrinkage prediction models for portland and non-portland cementitious materials. Adv Mater Sci Eng 2016(2016):2418219
Kovler K, Zhutovsky S (2006) Overview and future trends of shrinkage research. Mater Struct 39(9):827–847
Lura P, Jensen OM, van Breugel K (2003) Autogenous shrinkage in high-performance cement paste: an evaluation of basic mechanisms. Cem Concr Res 33(2):223–232
Grasley ZC, Leung CK (2011) Desiccation shrinkage of cementitious materials as an aging, poroviscoelastic response. Cem Concr Res 41(1):77–89
Badmann R, Stockhausen N, Setzer MJ (1981) The statistical thickness and the chemical potential of adsorbed water films. J Colloid Interface Sci 82(2):534–542
Wittmann F (1973) Interaction of hardened cement paste and water. J Am Ceram Soc 56(8):409–415
Bentz DP, Garboczi EJ, Quenard DA (1998) Modelling drying shrinkage in reconstructed porous materials: application to porous Vycor glass. Modell Simul Mater Sci Eng 6(3):211
Mackenzie J (1950) The elastic constants of a solid containing spherical holes. Proc Phys Soc Lond Sect B 63(1):2
Bentz DP (2005) Curing with shrinkage-reducing admixtures. Concrete Int Detroit 27(10):55
Vlahinić I, Jennings HM, Thomas JJ (2009) A constitutive model for drying of a partially saturated porous material. Mech Mater 41(3):319–328
Lura P, Lothenbach B, Miao C, Ye G, Chen H (2010) Influence of pore solution chemistryon shrinkage of cement paste. In: The 50-year teaching and research anniversary of prof sun wei on advances in civil engineering materials, pp 191–200
Jennings HM (2000) A model for the microstructure of calcium silicate hydrate in cement paste. Cem Concr Res 30(1):101–116
Ye H (2015) Creep mechanisms of calcium–silicate–hydrate: an overview of recent advances and challenges. Int J Concrete Struct Mater 9(4):453–462
Thiery M, Villain G, Dangla P, Platret G (2007) Investigation of the carbonation front shape on cementitious materials: effects of the chemical kinetics. Cem Concr Res 37(7):1047–1058
Houst Y (1989) Le retrait de carbonatation. Chantiers (Suisse) 20 (LTP-ARTICLE-2008-036):55-60
Acknowledgements
The authors would like to thank Prof. Farshad Rajabipour for his insightful thoughts and discussion regarding several topics discussed in this paper. The authors gratefully acknowledge the financial support from the US National Science Foundation (NSF) under Award CMMI #1265789. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, H., Radlińska, A. & Neves, J. Drying and carbonation shrinkage of cement paste containing alkalis. Mater Struct 50, 132 (2017). https://doi.org/10.1617/s11527-017-1006-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-017-1006-x