Abstract
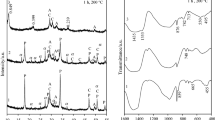
The pre-reaction temperature effects on the crystallization and micro-structure of xonotlite for C–S–H gels with Ca/Si ratio = 1 using a steam assisted crystallization process was investigated. The pre-reaction temperature affected the crystallinity, pore structure and silicate chain structure of C–S–H gels, thereby influencing the xonotlite micro-structure and formation kinetics. A higher Q 2/Q 1 Si peak intensity ratio were observed in C–S–H gels pre-reacted at 80 °C, indicating that a higher pre-reaction temperature can promote condensation reactions between the Si–OH units, and hence transforming of silicate chain structure from a single chain structure to a double chain structure. The XRD background intensities around 2Θ = 20°–35° resulting from the occurrence of amorphous C–S–H phase decreased as the pre-reaction temperature increased, suggesting that the pre-reaction temperature can increase the degree of structural order and crystallinity. More xonotlite were formed for C–S–H gels pre-reacted at 40 °C and 60 °C after reacting at 180 °C for 32 h and 190 °C for 16 h compared to the sample pre-reacted at 80 °C, due to the decrease of the solubility resulting from the increase of the average silicate chain length and the decrease of Ca/Si ratio after pre-reaction at 80 °C.











Similar content being viewed by others
References
Kubo K, Esumi M, Yamaguchi G (1976) Xonotlite heat-insulating materials with bulk density of 0.1. J Ceram Soc Jpn 84:23–28
Liu F, Zeng LK, Cao JX, Zhu B, An Yuan (2010) Hydrothermal synthesis of xonotlite fibers and investigation on their thermal property. Adv Mater Res 105–106:841–843
Taylor WH, Moorehead DR (1956) Lightweight calcium silicate hydrate-some mix and strength characteristics. Mag Concr Res 8:145–150
Moorehead DR, McCartney ER (1965) Hydrothermal formation of calcium silicate hydrates. J Am Ceram Soc 48:565–569
Li X, Chang J (2006) A novel hydrothermal route to the synthesis of xonotlite nano-fibers and investigation on their bioactivity. J Mater Sci 41:4944–4947
Kyritsis K, Hall C, Bentz DP, Meller N, Wilson MA (2009) Relationship between engineering properties, mineralogy, and microstructure in cement-based hydro-ceramic materials cured at 200°–350 °C. J Am Ceram Soc 92:694–701
Baltakys K, Jauberthie R (2009) Formation and stability of C-S-H(I) of various degrees of crystallinity in the Ca(OH)2/CaO-Hi-Sil-H2O system. Mater Sci Pol 27:1077–1089
Yanagisawa K, Feng Q, Yamasaki N (1997) Hydrothermal synthesis of xonotlite whiskers by ion diffusion. J Mater Sci Lett 16:889–891
Baspinar MS, Demir I, Kahraman E, Gorhan G (2014) Utilization potential of fly ash together with silica fume in autoclaved aerated concrete production. KSCE J Civ Eng 18:47–52
Liu F, Wang XD, Cao JX (2013) Effect of Na+ on xonotlite crystals in hydrothermal synthesis. Int J Miner Metall Mater 20:88–93
Siauciunas R, Baltakys K (2004) Formation of gyrolite during hydrothermal synthesis in the mixtures of CaO and amorphous or quartz. Cem Concr Res 34:2029–2036
Kunugida K, Tsukiyama K, Teramura S, Yuda S, Isu N, Shoji T, Takahashi H (1988) Direct formation of xonotlite fiber with continuous-type autoclave. Gypsum Lime 216:288–294
Arabi N, Jauberthie R, Sellami A (2013) Autoclaved sand-lime bricks: influence of addition of blast furnance slag on the formation of phases. Mater Struct 46:181–190
Sakiyama M, Asami T, Iwanaga T, Oshio Y, Oda M (1998) Method of manufacturing calcium silicate board. US patent 5,851,354
Williams SE, Plainfield N, Dunbar RB, Rademaker JC (1967) Manufacture of calcium silicate insulating products. US patent 3,352,746
Binkley ME (1955) Method of manufacture of heat insulating shapes. US patent 2,699,097
Cullity BD (1978) Elements of X-ray diffraction. Addison-Wesley, Reading
McFarlane AJ (2007) The synthesis and characterization of nano-structured calcium silicate. Doctoral Thesis, Victoria University of Wellington
Sasaki K, Masuda T, Ishida H, Mitsuda T (1997) Synthesis of calcium silicate hydrate with Ca/Si = 2 by mechanochemical treatment. J Am Ceram Soc 80:472–476
Cong X, Kirkpartrick RJ (1996) 29Si NMR study of the structure of calcium silicate hydrate. Adv Cem Based Mater 3:144–156
Richardson IG, Groves GW (1992) Model for the composition and structure of calcium silicate hydrate (C-S-H) gel in hardened tricalcium silicate pastes. Cem Concr Res 22:1001–1010
Okada Y, Ishida H, Mitsuda T (1994) 29Si NMR spectroscopy of silicate anions in hydrothermal formed C-S-H. J Am Ceram Soc 77:765–768
Wieker W, Grimmer AR, Winkler A, Magi M, Tarmk M, Lippmaa E (1982) Solid state high resolution 29Si NMR spectroscopy of synthetic 14Å, 11Å and 9Å tobermorites. Cem Concr Res 12:333–339
García-Lodeiro I, Fernández-Jiménez A, Sobrados I, Sanz J, Palomo A (2012) C-S-H gels: interpretation of 29Si MAS-NMR spectra. J Am Ceram Soc 95:1440–1446
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemienewska T (1985) Reporting physisorption data for gas-solid systems. Pure Appl Chem 57:603–619
Glasser FP, Hong SY (2003) Thermal treatment of C-S-H Gel at 1 bar H2O pressure up to 200 °C. Cem Concr Res 33:271–279
Grutzeck MW (1999) A new model for the formation of calcium silicate hydrate (C-S-H). Mater Res Innov 13:160–170
Houston JR, Maxwell RS, Carroll SA (2009) Transformation of meta-stable calcium silicate hydrates to tobermorite: reaction kinetics and molecular structure from XRD and NMR spectroscopy. Geochem Trans 10:1–14
Shaw S, Clark SM, Henderson CMB (2000) Hydrothermal formation of the calcium silicate hydrates, tobermorite(Ca5Si6O16(OH)2·4H2O) and xonotlite (Ca6Si6O17(OH)2): an in situ synchrotron study. Chem Geol 167:129–140
Kikuma J, Tsunashima M, Ishikawa T, Matsuno SY, Ogawa A, Matsui K, Sato M (2010) In situ time-resolved X-ray diffraction of tobermorite formation process under autoclave condition. J Am Ceram Soc 93:2667–2674
Matsui K, Kikuma J, Tsunashima M, Ishikawa T, Matsuno SY, Ogawa A, Sato M (2010) In situ time-resolved X-ray diffraction of tobermorite formation in autoclaved aerated concrete: influence of silica source reactivity and Al addition. Cem Concr Res 41:510–519
Jenning HM (1986) Aqueous solubility relationships for two types of calcium silicate hydrate. J Am Ceram Soc 69:614–618
Mitsuda T, Sasaki K, Ishida H (1992) Phase evolution during autoclaving process of aerated concrete. J Am Ceram Soc 75:1858–1863
Liu F, Li T, Wang XD, Cao JX (2013) Effect of the ratio of silica source on ultra-light calcium silicate material in situ strengthened xonotlite whiskers. Adv Mater Res 648:131–134
Acknowledgments
This work was financially sponsored by the Headquarters of University Advancement at the National Cheng Kung University, which is sponsored by the Ministry of Education, Taiwan, ROC and Jiadah Chemical Co. Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsiang, HI., Chen, WS. & Huang, WC. Pre-reaction temperature effect on C–S–H colloidal properties and xonotlite formation via steam assisted crystallization. Mater Struct 49, 905–915 (2016). https://doi.org/10.1617/s11527-015-0547-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1617/s11527-015-0547-0