Abstract
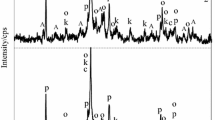
A C–S–H series with calcium–silicon ratio 0.6–3.0 was synthesized by pozzolanic reaction. Phase composition, nanostructural and morphological characteristics were determined using XRD, XRF, SEM and 29Si NMR. Most of the samples were phase-pure, poorly crystalline C–S–H. Significant changes in the nanostructure of the C–S–H samples were observed when the calcium–silicon ratio reached values of 0.8, 1.0 and 1.5. At calcium–silicon ratio 0.8 the basal XRD peak began to develop, crosslinking between layers was seen below this ratio but not above, and there was a substantial decrease in mean silica chain length at this ratio. At calcium–silicon ratio 1.0 there was a pronounced microstructural change from granular to reticular and another substantial decrease in mean chain length (indicated by an abrupt increase in the Q1 peak intensity and decrease in the Q2 peak intensity). At calcium–silicon ratio 1.5 the basal XRD peak began to diminish again, the mean silica chain length decreased further, and isolated tetrahedra (Q0) were observed.






Similar content being viewed by others
References
Taylor HFW (1997) Cement chemistry, 2nd edn. Thomas Telford, London
Richardson IG (1999) The nature of C–S–H in hardened cements. Cem Concr Res 29:1131–1147
Chen JJ (2003) The nanostructure of calcium silicate hydrate. PhD thesis, Northwestern University
Cong XD (1994) 29Si and 17O nuclear magnetic resonance investigation of the structure of calcium–silicate–hydrate. PhD thesis, University of Illinois, Urbana-Champaign
Locher FW (1966) Stoichiometry of tricalcium silicate hydration, Special Report. Highway Research Board, Washington, DC, pp 300–308
Odler I, Skalny J (1973) Hydration of tricalcium silicate at elevated temperature. J Appl Chem Biotechnol 23:661–667
Diamond S (1976) C/S mole ratio of C–S–H gel in a mature C3S paste as determined by EDXA. Cem Concr Res 16:413–416
Gard JA, Mohan K, Taylor HFW, Cliff G (1980) Analytical electron microscopy of cement pastes I, tricalcium silicate pastes. J Am Ceram Soc 63:336–337
Lachowski EE, Mohan K, Taylor HFW, Lawrence CD, Moore AE (1981) Analytical electron microscopy of cement pastes: III pastes hydrated for long times. J Am Ceram Soc 64:319–321
Mohan K, Taylor HFW (1981) Analytical electron microscopy of cement pastes: IV dicalcium silicate pastes. J Am Ceram Soc 64:717–719
Richardson IG, Groves GW (1993) Microstructure and microanalysis of hardened ordinary Portland cement pastes. J Mater Sci 28:265–277
Kumar R, Kumar S, Badjena S, Mehrotra SP (2005) Hydration of mechanically activated granulated blast furnace slag. Metall Mater Trans B 36(12):873–883
Girão CAV, Richardson IG, Porteneuve CB, Brydson RMD (2007) Composition, morphology and nanostructure of C–S–H in white Portland cement pastes hydrated at 55 °C. Cem Concr Res 37:1571–1582
Luke K, Lachowski E (2008) Internal composition of 20-year-old fly ash and slag-blended ordinary Portland cement pastes. J Am Ceram Soc 91(12):4084–4092
Taylor HFW (1950) Hydrated calcium silicates, part I compound formation at ordinary temperatures. J Chem Soc 3682–3690. doi:10.1039/JR9500003690
Taylor HFW (1968) The calcium silicate hydrate. In: Proceedings of the 5th Symposium Chemistry Cement, Tokyo, II, pp 1–26
Taylor HFW (1990) Cement chemistry. Academic Press, London
Gard JA, Taylor HFW (1976) Calcium silicate hydrate(II) (C–S–H(II)). Cem Concr Res 6:667–678
Taylor HFW (1986) Proposed structure for calcium silicate hydrate gel. J Am Ceram Soc 69(6):464–467
Taylor HFW (1993) Nanostructure of C–S–H: current status. Adv Cem Bas Mat 1:38–46
Bell GMM, Bensted J, Glasser FP, Lachowshi EE, Roberts DR, Taylor MJ (1990) Study of calcium silicate by solid state high resolution 29Si nuclear magnetic resonance. Adv Cem Res 3:23–38
Cong XD, Kirkpatrick RJ (1996) 29Si MAS NMR study of the structure of calcium silicate hydrate. Adv Cem Based Mater 3:144–156
Grutzeck M, Benesi A, Fanning B (1989) Silicon-29 magic angle spinning nuclear magnetic resonance study of calcium silicate hydrates. J Am Ceram Soc 72(4):665–668
Lippmass E, Mägi M, Tarmak M, Wieker W, Grimmer AR (1982) A high resolution 29Si NMR study of the hydration of tricalcium silicate. Cem Concr Res 12:597–602
Young JF (1988) Investigations of calcium silicate hydrates structure using silicon-29 nuclear magnetic resonance spectroscopy. J Am Ceram Soc 71:C-118–C-120
Rodger SA, Groves GW, Clayden NJ, Dodson CM (1988) Hydration of tricalcium silicate followed by 29Si NMR with cross-polarization. J Am Ceram Soc 71:91–96
Cong XD, Kirkpatrick RJ (1993) 17O and 29Si MAS NMR study of β-C2S hydration and the structure of calcium–silicate hydrates. Cem Concr Res 23:1065–1077
Fernandez L, Alonso C, Andrade C, Hidalgo A (2008) The interaction of magnesium in hydration of C3S and CSH formation using 29Si MAS-NMR. J Mater Sci 43:5772–5783
Barnes JR, Clague ADH, Clayden NJ, Dobson CM, Hayes CJ, Groves GW, Rodger SA (1985) Hydration of Portland cement followed by 29Si solid-state NMR spectroscopy. J Mater Sci Lett 4(10):1293–1295
Beaudoin JJ, Raki L, Alizadeh R (2009) A 29Si MAS NMR study of modified C–S–H nanostructures. Cem Concr Compos 31:585–590
Tennis PD, Jennings HM (2000) A model for two types of calcium silicate hydrate in the microstructure of Portland cement pastes. Cem Concr Res 30:855–863
Diamond S (1976) Cement paste microstructure: an overview at several levels, in Hydraulic cement pastes: their structure and properties. Wexham Springs. pp 1–30
Richardson IG (2008) The calcium silicate hydrates. Cem Concr Res 38:137–158
Stucke MS, Majumdar AJ (1977) The morphology and composition of an immature C3S paste. Cem Concr Res 7:711–718
Chatterji S, Jeffrey JW (1962) Studies of early stages of paste hydration of cement compounds I. J Am Ceram Soc 45(11):536–543
Lawrence FV Jr, Young JF (1973) Studies on the hydration of tricalcium silicate pastes: I. Scanning electron microscopic examination of microstructural features. Cem Concr Res 3(2):149–161
Grudemo A (1960) The microstructure of hardened cement paste. In: Proceedings of the Fourth International Symposium on the Chemistry of Cement, Washington, II, pp 615–658
Richardson IG, Cabrera JG (2000) The nature of C–S–H in model slag-cements. Cem Concr Compos 22:259–266
Diamond S, Bonen D (1993) Microstructure of hardened cement paste—a new interpretation. J Am Ceram Soc 76(12):2993–2999
Jennings HM, Dalgleish BJ, Pratt PL (1981) Morphological development of hydrating tricalcium silicate as examined by electron microscopy. J Am Ceram Soc 64(10):567–572
Ciach TD, Gillott JE, Swenson EG, Sereda PJ (1971) Microstructure of calcium silicate hydrates. Cem Concr Res 1:13–25
Jennings HM, Tennis PD (1994) Model for the developing microstructure in Portland Cement Pastes. J Am Ceram Soc 77(12):3161–3172
Jennings HM (2000) A model for the microstructure of calcium silicate hydrate in cement paste. Cem Concr Res 30:101–116
Klur I, Pollet B, Virlet J, Nonat A (1996) C–S–H structure evolution with calcium content by multinuclear NMR. In: Colombet P, Grimmer A-R, Zanni H, Sozzani P (eds) Nuclear magnetic resonance spectroscopy of cement-based materials. Springer, Berlin, pp 119–141
Farmer VC, Jeevaratnam J, Speakman K, Taylor HFW (1966) Thermal decomposition of 14 Å tobermorite from crestmore. In: Symposium on structure of Portland cement paste and concrete (special report 90). Highway Research Board, Washington, DC, pp 291–298
Hamid SA (1981) The crystal structure of the 11 Å natural tobermorite Ca2.25[Si3O7.5(OH)1.5] 1H2O. Zeit Kristall 154:189–198
Gard JA, Taylor HFW, Cliff G, Lorimer GW (1977) A reexamination of jennite. Am Miner 62:365–368
Lippmass E, Mägi M, Samoson A, Engelhardt G, Grimmer A-R (1980) Structural studies of silicates by solid-state high-resolution 29Si NMR. J Am Chem Soc 102:4889–4893
Maciel GE, Sindorf DW (1980) Silicon-29 NMR study of the surface of silica gel by cross polarization and magic-angle spinning. J Am Chem Soc 102:7606–7607
Cong XD, Kirkpatrik RJ (1993) 29Si MAS NMR spectroscopic investigation of alkali silica reaction product gels. Cem Concr Res 23:811–823
Chen JJ, Thomas JJ, Taylor HFW, Jennings HM (2004) Solubility and structure of calcium silicate hydrate. Cem Concr Res 34:1499–1519
Macphee DE, Lachowski EE, Glasser FP (1988) Polymerization effects in C-S–H: implications for Portland cement hydration. Adv Cem Res 1:131–137
Wieker W, Grimmer A-R, Winkler A, Mägi M, Tarmak M, Lippmaa E (1982) Solid-state high-resolution 29Si NMR spectroscopy of synthetic 14 Å, 11 Å, and 9 Å tobermorites. Cem Concr Res 12:333–339
Sato H, Grutzeck M (1992) Effect of starting materials on the synthesis of tobermorite. Mater Res Soc Symp Proc 245:235–240
Popova A, Geoffroy G, Renou-Gonnord MF, Faucon P, Gartner E (2000) Interactions between polymeric dispersants and calcium silicate hydrates. J Am Ceram Soc 83(10):2556–2560
Sherriff BL, Grundy HD (1988) Calculations of 29Si MAS NMR chemical shift from silicate mineral structure. Nature 332:819–822
Brough AR, Dobson CM, Richardson IG, Groves GW (1994) Application of selective 29Si isotopic enrichment to studies of the structure of calcium silicate hydrate (C–S–H) gels. J Am Ceram Soc 77:593–596
Richardson IG, Groves GW (1992) Models for the composition and structure of calcium silicate hydrate (C–S–H) gel in hardened tricalcium silicate pastes. Cem Concr Res 22:1001–1010
Acknowledgments
The work presented in this paper was supported by the National Basic Research Program of China (973 Program, No. 2009CB623201) and Natural Science Foundation of China (No. 51072150). We would like to thank Xiaogang Zhao for some experimental help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, Y., Lu, L., Struble, L.J. et al. Effect of calcium–silicon ratio on microstructure and nanostructure of calcium silicate hydrate synthesized by reaction of fumed silica and calcium oxide at room temperature. Mater Struct 47, 311–322 (2014). https://doi.org/10.1617/s11527-013-0062-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1617/s11527-013-0062-0