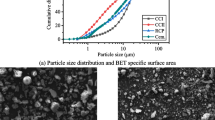
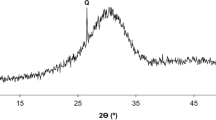
Abstract
Three series of fine limestone aggregate, alkali-activated blast furnace slag (AAS) concretes were fabricated and tested; two through activation with waterglass/NaOH solution, of which one included NaCl as a retarding agent, and one activated by Na2CO3. Each of these series was made up of three formulae containing different amounts of Al2O3. The compressive strengths of the series activated by waterglass/NaOH after 28 days were ≈65 ± 5.3 MPa, a 22% increase compared to previously reported formulae containing no additional Al2O3. Increasing the amount of Al2O3 did not further increase strength, however. The Na2CO3-activated formulae had strengths of ≈35 ± 3 MPa after 28 days, representing no increase in strength over formulae not containing Al2O3 previously reported. X-ray diffraction showed the main binding phase to be calcium silicate hydrate (C–S–H) gel, as is commonly found in ordinary Portland cement (OPC). Fourier transform infrared spectroscopy showed little difference from the previously reported results for formulae not containing Al2O3 and strongly resemble the spectra reported elsewhere for C–S–H. Electron microscopy, coupled with energy dispersive spectroscopy, showed the cementing phase to be a single homogenous phase—not a mixed system of geopolymer and C–S–H gel—with a lower volume fraction of unreacted slag than formulae without Al2O3. The reason for the increase in strength of Al2O3-containing formulae is unclear, but is unlikely to be ascribed to the formation of large amounts of ‘geopolymers’ and may be related to a possible increase in reaction temperature of between 2 and 5°C, depending on amount of additive.






Similar content being viewed by others
Notes
Cement chemists use single letters to represent oxides: C=CaO, S=SiO2, A=Al2O3, F=Fe2O3, H=H2O and so on. Calcium-silicate hydrates are abbreviated C–S–H due to the possible existence of a range of compositions; therefore using exact stoichiometry would be both difficult to read and inaccurate.
References
Brough AR, Atkinson A (2002) Sodium silicate based, alkali activated slag mortars, part I: strength, hydration and microstructure. Cem Concr Res 32:865
Brough AR, Holloway M, Sykes J, Atkinson A (2000) Sodium silicate based alkali activated slag mortars, part II: the retarding effect of additions of sodium chloride or malic acid. Cem Concr Res 30:1375
Davidovits J (1991) Geopolymers: inorganic polymeric new materials. J Therm Anal 37:1633
Lecomte I, Henrist C, Liegéois M, Maseri F, Rulmont A, Cloots R (2006) (Micro)-structural comparison between geopolymers, alkali-activated slag cement and Portland cement. J Eur Ceram Soc 26:8
Li Z, Ding Z, Zhang Y (2003) Development of sustainable cementitious materials. International workshop on sustainable development and concrete technology
Palomo A, Grutzeck MW, Blanco MT (1999) Alkali-activated fly ashes: a cement for the future. Cem Concr Res 29:6
Roy DM (1999) Alkali activated cements: opportunities and challenges. Cem Concr Res 29:249
Talling B, Brandstetr J (1989) Present state and future of alkali activated slag concretes. In: 3rd international conference on fly ash, silica fume, slag, and natural Pozzolans in concrete. Trondheim, Norway
Teoreanu I, Volceanov A, Stoleriu S (2005) Non Portland cements and derived materials. Cem Concr Compos 27:10
Wang S-D, Scrivener KL, Pratt PL (1994) Factors affecting the strength of alkali-activated slag. Cem Concr Res 24:10
Richardson IG, Groves GW (1992) Microstructure and microanalysis of hardened cement pastes involving ground granulated blast-furnace slag. J Mater Sci 27:8
Puertas F, Fernández-Jiminéz A (2003) Mineralogical and microstructural characterization of alkali-activated fly ash/slag pastes. Cem Concr Compos 25:287
Puertas F, Martinez-Ramierz S, Alonso S, Vazquez T (2000) Alkali-activated fly ash/slag cement strength behaviour and hydration products. Cem Concr Res 30:7
Collins F, Sanjayan JG (2001) Microcracking and strength development of alkali activated slag concrete. Cem Concr Compos 23:7
Dongxu L, Xuequan W, Jinlin S, Yujiang W (2000) The influence of compound admixtures on the properties of high content slag cement. Cem Concr Res 30:5
Douglas E, Bilodeau A, Malhotra VM (1993) Properties and durability of alkali-activated slag concrete. ACI Mater J 89:509
Kalyoncu RS (2001) Slag—iron and steel. USGS Minerals Yearbook 2001 2001:7
Gartner E (2004) Industrially interesting approaches to “Low CO2” cements. Cem Concr Res 24:9
Worrell E, Price L, Martin N, Hendriks C, Meida LO (2001) Carbon dioxide emissions from the global cement industry. Annu Rev Energy Environ 26:303
Bogue RH (1947) The chemistry of Portland cement. Reinhold Publishing Corp, New York
Ŝkvára F (2007) Alkali activated materials or geopolymers? Ceramics 51:173
Cheng TW, Chiu JP (2002) Fire resistant geopolymer produced by granulated blast furnace slag. Miner Eng 16:205
Davidovits J (2002) 30 Years of successes and failures in geopolymer applications. Market trends and potential breakthroughs. In: Geopolymer 2002 conference. Melbourne, Australia
Zhang Y, Wei S, Qianli C, Lin C (2007) Synthesis and heavy metal immobilization behaviors of slag based geopolymer. J Hazard Mater 143:206
Zosin AP, Priimak TI, Avsaragov KB (1998) Geopolymer materials based on magnesia-iron slags for normalization and storage of radioactive wastes. Atomic Energy 85:78
van Deventer JSJ, Lukey GC, Yip CK (2005) The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem Concr Res 35:9
van Deventer JSJ, Yip CK (2003) Microanalysis of calcium silicate hydrate gel formed within a geopolymeric binder. J Mater Sci 28:9
Sakulich AR, Anderson E, Schauer C, Barsoum MW (2009) Mechanical and microstructural characterization of an alkali-activated slag/limestone fine aggregate concrete. Constr Build Mater 23(8):2951–2957
Davidovits J (2008) Geopolymer chemistry and applications. The Geopolymer Institute, Saint-Quentin
Colthup NB, Wiberley SE, Daly LH (1975) Introduction to infrared and Raman spectroscopy. Academic Press, New York
Silverstein RM, Webster FX (1998) Spectrometric identification of organic compounds. Wiley, New York
Wang SD, Scrivener KL (1995) Hydration products of alkali activated slag cement. Cem Concr Res 25:561
Duxson P, Fernández-Jiménez A, Provis JL, Lukey GC, Palomo A, van Deventer JSJ (2007) Geopolymer technology: the current state of the art. J Mater Sci 42:16
Rowles M, O’Conner B (2003) Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of metakaolinite. J Mater Chem 13:1161
Acknowledgment
This work was partially funded by NSF (DMR 0503711). The help of Dr. Eva Jud Sierra and Sean Miller of Drexel University is also appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakulich, A.R., Anderson, E., Schauer, C.L. et al. Influence of Si:Al ratio on the microstructural and mechanical properties of a fine-limestone aggregate alkali-activated slag concrete. Mater Struct 43, 1025–1035 (2010). https://doi.org/10.1617/s11527-009-9563-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1617/s11527-009-9563-2