Abstract
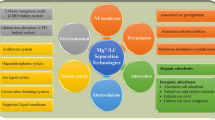
Geothermal fluids have the potential as important sources of precious minerals and metals. There are several hydrometallurgical techniques by which geothermal fluid solutions can be processed to extract and purify metals and minerals such as potassium, manganese, zinc, and lithium. The primary methods for extraction of salt and base metals from geothermal water include precipitation, electrodialysis, reverse osmosis, adsorption, electrochemical intercalation, and ion exchange. Among several methods discussed so far membrane and adsorption methods can be one of the suitable methods for extraction of salt and base metals, respectively. The article also summarizes various mathematical modeling used to study dynamic behavior and kinetics of column adsorption. The three most widely used column models, i.e., Thomas, BDST, and Yoon–Nelson are discussed herein, that help to estimate the adsorption capacity and intensity giving an overview of mechanism and forces responsible for column sorption process. The elaborate discussion on mechanistic forces and factors responsible for metal extraction by sorption makes this review significant and preferable. Therefore, the article aims to provide deep insights and a quick overview of salt and base metal sources, their extraction processes, column sorption dynamics, kinetic modeling, and mechanisms in one sight.
Graphical Abstract
Work flow for Base metal Extraction from geothermal water.






Similar content being viewed by others
References
S. Kumar, S.K. Gupta, M. Rawat, Resources and utilization of geothermal energy in India: an eco–friendly approach towards sustainability. Mater. Today: Proc. 26, 1660–1665 (2020). https://doi.org/10.1016/j.matpr.2020.02.347
Sharma, S., Aggarwal, S., Baghel, Y.K., Kumar, A. and Kesari, J.P, Problems of Scaling and Corrosion in Geothermal Water: Indian Scenario (2019)
M. Soltani, F. MoradiKashkooli, A.R. Dehghani-Sanij, A. Nokhosteen, A. Ahmadi-Joughi, K. Gharali, S.B. Mahbaz, M.B. Dusseault, A comprehensive review of geothermal energy evolution and development. Int. J. Green Energy 16(13), 971–1009 (2019)
R. Pell, L. Tijsseling, K. Goodenough, F. Wall, Q. Dehaine, A. Grant, D. Deak, X. Yan, P. Whattoff, Towards sustainable extraction of technology materials through integrated approaches. Nat. Rev. Earth Environ. 2(10), 665–679 (2021)
M. Fitzner, A. Fricke, M. Schreiner, S. Baldermann, Utilization of regional natural brines for the indoor cultivation of salicorniaeuropaea. Sustainability 13(21), 12105 (2021). https://doi.org/10.3390/su132112105
P. Meshram, B.D. Pandey, Perspective of availability and sustainable recycling prospects of metals in rechargeable batteries–a resource overview. Resour. Policy 60, 9–22 (2019)
M. Šolić, S. Maletić, M. KraguljIsakovski, J. Nikić, M. Watson, Z. Kónya, J. Tričković, Comparing the adsorption performance of multiwalled carbon nanotubes oxidized by varying degrees for removal of low levels of copper, nickel, and chromium (VI) from aqueous solutions. Water 12(3), 723 (2020)
W.T. Stringfellow, P.F. Dobson, Technology for the recovery of lithium from geothermal brine. Energies 14(20), 6805 (2021)
D.L. Gallup, Geochemistry of geothermal fluids and well scales, and potential for mineral recovery. Ore Geol. Rev. 12(4), 225–236 (1998). https://doi.org/10.1016/S0169-1368(98)00004-3
Schulz, K.J. ed., Critical mineral resources of the United States: economic and environmental geology and prospects for future supply. Geological Survey (2017)
C. Grosjean, P.H. Miranda, M. Perrin, P. Poggi, Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry. Renew. Sustain. Energy Rev. 16(3), 1735–1744 (2012). https://doi.org/10.1016/j.rser.2011.11.023
V. Flexer, C.F. Baspineiro, C.I. Galli, Lithium recovery from brines: A vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total Environ. 639, 1188–1204 (2018). https://doi.org/10.1016/j.scitotenv.2018.05.223
Hund, K., La Porta, D., Fabregas, T.P., Laing, T. and Drexhage, J., Minerals for climate action: the mineral intensity of the clean energy transition. World Bank, 73 (2020)
H. Kaasalainen, A. Stefánsson, The chemistry of trace elements in surface geothermal waters and steam Iceland. Chem. Geol. 330, 60–85 (2012)
Neupane, G. and Wendt, D.S., February. Assessment of mineral resources in geothermal brines in the US. In Proceedings of the 42nd Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, CA, USA pp. 13–15 (2017)
Z.S. Cetiner, Ö. Doğan, G. Özdilek, P.Ö. Erdoğan, Toward utilizing geothermal waters for cleaner and sustainable production: Potential of Li recovery from geothermal brines in Turkey. Int. J. Glob. Warm. 7(4), 439–453 (2015)
Svalova, V., April. Mineral extraction from brines and geothermal resources complex use in Russia. In Proceedings of World Geothermal Congress (2010)
Mroczek, E., Climo, M., Li, Y., Evans, D., Carey, B. and Gao, W., April. From waste to wealth: mineral extraction from geothermal brines. In Proceedings World Geothermal Congress (2015)
Y. Ghalavand, M.S. Hatamipour, A. Rahimi, A review on energy consumption of desalination processes. Desalin. Water Treat. 54(6), 1526–1541 (2015). https://doi.org/10.1080/19443994.2014.892837
H.T. Do Thi, T. Pasztor, D. Fozer, F. Manenti, A.J. Toth, Comparison of desalination technologies using renewable energy sources with the life cycle, PESTLE, and multi-criteria decision analyses. Water 13(21), 3023 (2021). https://doi.org/10.3390/w13213023
Al Washahi, M. and Gopinath, A.S., May. Techno Economical Feasibility Analysis of Solar Powered RO Desalination in Sultanate of Oman. In 2017 9th IEEE-GCC Conference and Exhibition (GCCCE), p. 1–9 (2017)
M.A. Abdelkareem, M.E.H. Assad, E.T. Sayed, B. Soudan, Recent progress in the use of renewable energy sources to power water desalination plants. Desalination 435, 97–113 (2018). https://doi.org/10.1016/j.desal.2017.11.018
Nugroho Agung Pambudi, Jamiatul Yusafiadi, Muhammad Kunta Biddinika, Yuyun Estriyanto, Alfan Sarifudin, An experimental investigation of salt production improvement by spraying and heating. Case Stud Therm. Eng. 30, 101739 (2022)
B. Tomaszewska, A. Szczepański, Possibilities for the efficient utilization of spent geothermal waters. Environ. Sci. Pollut. Res. 21, 11409–11417 (2014)
R. Ibanez, A. Perez-Gonzalez, J. Pinedo, P. Gomez, A.M. Urtiaga, I. Ortiz, Review of direct discharge and recovery of reverse osmosis concentrates, in Geothermal Water Management. ed. by J. Bundschuh, B. Tomaszewska (CRC Press, Boca Raton, 2018), pp.233–254
M. Bodzek, K. Konieczny, Membrane techniques in the treatment of geothermal water for fresh and potable water production, in Geothermal Water Management. ed. by J. Bundschuh, B. Tomaszewska (CRC Press, Boca Raton, 2018), pp.157–231
B. Tomaszewska, A new approach to the utilization of concentrates obtained during geothermal water desalination. Desalin. Water Treat. 128, 407–413 (2018)
N. Voutchkov, Considerations for selection of seawater filtration pretreatment system. Desalination 261(3), 354–364 (2010)
M.K. Wafi, N. Hussain, O. El-Sharief Abdalla, M.D. Al-Far, N.A. Al-Hajaj, K.F. Alzonnikah, Nanofiltration as a cost-saving desalination process. SN Appl. Sci. 1, 1–9 (2019)
H. Kristmannsdottir, H. Armannsson, Environmental aspects of geothermal energy utilization. Geothermics 32(4–6), 451–461 (2003). https://doi.org/10.1016/j.jhazmat.2007.07.041
X. Su, Y. Song, T. Li, C. Gao, Effect of feed water characteristics on nanofiltration separating performance for brackish water treatment in the Huanghuai region of China. J. Water Process Eng. 19, 147–155 (2017)
R. Clayton, Desalination for Water Supply FR/R0013 (35p) (Review of Current Knowledge, Foundation for Water Research, UK, 2006)
F. Van der Ham, G.J. Witkamp, J. De Graauw, G.M. Van Rosmalen, Eutectic freeze crystallization simultaneous formation and separation of two solid phases. J. Cryst. Growth 198, 744–748 (1999)
Kasedde, H., Kirabira, J., Bäbler, M., Tilliander, A. and Jonsson, S., A state-of-the-art paper on improving salt extraction from lake kale raw materials in Uganda (2012)
O. Kılıc, A.M. Kilic, Recovery of salt co-products during the salt production from brine. Desalination 186(1–3), 11–19 (2005)
G. Dermont, M. Bergeron, G. Mercier, M. Richer-Laflèche, Soil washing for metal removal: a review of physical/chemical technologies and field applications. J. Hazard. Mater. 152(1), 1–31 (2008)
V.G. Gude, N. Nirmalakhandan, S. Deng, Renewable and sustainable approaches for desalination. Renew. Sustain. Energy Rev. 14(9), 2641–2654 (2010). https://doi.org/10.1016/j.rser.2010.06.008
Y. Tanaka, R. Ehara, S. Itoi, T. Goto, Ion-exchange membrane electrodialytic salt production using brine discharged from a reverse osmosis seawater desalination plant. J. Membr. Sci. 222(1–2), 71–86 (2003)
V.R. Ginde, C. Chieh, An apparatus for desalination with ion exchange resins. Desalination 10(4), 309–317 (1972). https://doi.org/10.1016/S0011-9164(00)80001-6
Kobuchi, Y., Terada, Y. and Tani, Y., The first salt plant in the Middle East using electrodialysis and ion exchange membranes. In Sixth International Symposium on Salt ll, pp. 541–555 (1983)
A.E. Ghaly, M. Verma, Desalination of saline sludges using ion-exchange column with zeolite. Am. J. Environ Sci. 4(4), 388 (2008)
N. Kabay, I. Yılmaz, S. Yamac, S. Samatya, M. Yuksel, U. Yuksel, M. Arda, M. Sağlam, T. Iwanaga, K. Hirowatari, Removal and recovery of boron from geothermal wastewater by selective ion exchange resins. I. Laboratory tests. React. Funct. Polym. 60, 163–170 (2004)
Z.S. Cetiner, O. Dogan, G. Ozdilek, P.O. Erdogan, Toward utilizing geothermal waters for cleaner and sustainable production: potential of Li recovery from geothermal brines in Turkey. Int. J. Glob. Warm. 7(4), 439–453 (2015). https://doi.org/10.1504/IJGW.2015.070045
Farley, E.P., Watson, E.L., MacDonald, D.D., Bartlett, R.W. and Krishnan, G.N., Recovery of heavy metals from high salinity geothermal brine. Open file report (final) 29 Sep 78–28 Oct 80 (No. PB-81–222218). SRI International, Menlo Park, CA (USA) (1980)
Pauwels, H., Brach, M. and Fouillac, C., ‘Lithium recovery from geothermal waters of Cesano (Italy) and Cronembourg (Alsace, France)’, 12th New Zealand Geothermal Workshop, pp. 117–123 (1990)
Um, N. and Hirato, T., A Study on Lithium Recovery from Seawater: Separation of Lithium from Hydrochloric Acid Solutions Containing CaCl2, MgCl2, MnCl2, NaCl, KCl, and LiCl. In Zero-Carbon Energy Kyoto 2012: Special Edition of the Joint Symposium “Energy Science in the Age of Global Warming” of the Kyoto University Global COE Program and the JGSEE/CEE-KMUTT, pp. 149–154 (2013)
A. Yaksic, J.E. Tilton, Using the cumulative availability curve to assess the threat of mineral depletion: the case of lithium. Resour. Policy 34(4), 185–194 (2009)
C.A. Quist-Jensen, A. Ali, S. Mondal, F. Macedonio, E. Drioli, A study of membrane distillation and crystallization for lithium recovery from high-concentrated aqueous solutions. J. Membr. Sci. 505, 167–173 (2016)
C. Jiang, Y. Wang, Q. Wang, H. Feng, T. Xu, Production of lithium hydroxide from lake brines through electro–electrodialysis with bipolar membranes (EEDBM). Ind. Eng. Chem. Res. 53(14), 6103–6112 (2014)
S. Al-Amshawee, M.Y.B.M. Yunus, A.A.M. Azoddein, D.G. Hassell, I.H. Dakhil, H.A. Hasan, Electrodialysis desalination for water and wastewater: a review. Chem. Eng. J. 380, 122231 (2020). https://doi.org/10.1016/j.cej.2019.122231
T. Hoshino, Preliminary studies of lithium recovery technology from seawater by electrodialysis using ionic liquid membrane. Desalination 317, 11–16 (2013). https://doi.org/10.1016/j.desal.2013.02.014
Zhongwei, Z.H.A.O. and Xuheng, L.I.U., Central South University, Method and device for extracting and enriching lithium. U.S. Patent 9,062,385 (2015)
J.W. An, D.J. Kang, K.T. Tran, M.J. Kim, T. Lim, T. Tran, Recovery of lithium from Uyunisalar brine. Hydrometallurgy 117, 64–70 (2012). https://doi.org/10.1016/j.cej.2019.122231
Ventura, S., Bhamidi, S., Hornbostel, M., Nagar, A. and Perea, E., Selective recovery of metals from geothermal brines (No. DOE-SRI-6747). SRI International, Menlo Park, CA (United States) (2016)
Peerawattuk, I. and Bobicki, E.R., Lithium Extraction and Utilization: A Historical Perspective. In Extraction 2018: Proceedings of the First Global Conference on Extractive Metallurgy, pp. 2209–2224. Springer International Publishing, (2018)
L. Li, V.G. Deshmane, M.P. Paranthaman, R.R. Bhave, B.A. Moyer, S. Harrison, Lithium recovery from aqueous resources and batteries: a brief review. Johns. Matthey Technol. Rev. (2018). https://doi.org/10.1595/205651317X696676
M. Kapur, M.K. Mondal, Design and model parameters estimation for fixed–bed column adsorption of Cu (II) and Ni (II) ions using magnetized saw dust. Desalin. Water Treat. 57(26), 12192–12203 (2016). https://doi.org/10.1080/19443994.2015.1049961
Y.C. Lo, C.L. Cheng, Y.L. Han, B.Y. Chen, J.S. Chang, Recovery of high-value metals from geothermal sites by biosorption and bioaccumulation. Biores. Technol. 160, 182–190 (2014)
G. Zante, M. Boltoeva, A. Masmoudi, R. Barillon, D. Trebouet, Highly selective transport of lithium across a supported liquid membrane. J. Fluorine Chem. 236, 109593 (2020)
B. Swain, Recovery and recycling of lithium: a review. Sep. Purif. Technol. 172, 388–403 (2017)
H. Wang, J. Cui, M. Li, Y. Guo, T. Deng, X. Yu, Selective recovery of lithium from geothermal water by EGDE cross-linked spherical CTS/LMO. Chem. Eng. J. 389, 124410 (2020)
P. Meshram, B.D. Pandey, T.R. Mankhand, Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: a comprehensive review. Hydrometallurgy 150, 192–208 (2014)
J.F. Song, L.D. Nghiem, X.M. Li, T. He, Lithium extraction from Chinese salt-lake brines opportunities, challenges, and future outlook. Environ. Sci.: Water Res. Technol. 3(4), 593–597 (2017)
X. Li, Y. Mo, W. Qing, S. Shao, C.Y. Tang, J. Li, Membrane-based technologies for lithium recovery from water lithium resources: a review. J. Membr. Sc. 591, 117317 (2019). https://doi.org/10.1016/j.memsci.2019.117317
X. Liu, X. Chen, L. He, Z. Zhao, Study on extraction of lithium from salt lake brine by membrane electrolysis. Desalination 376, 35–40 (2015)
C. Liu, Y. Li, D. Lin, P.C. Hsu, B. Liu, G. Yan, S. Chu, Lithium extraction from seawater through pulsed electrochemical intercalation. Joule 4(7), 1459–1469 (2020)
G. Yan, M. Wang, G.T. Hill, S. Zou, C. Liu, Defining the challenges of Li extraction with olivine host: the roles of competitor and spectator ions. Proc. Natl. Acad. Sci. 119(31), e2200751119 (2022)
S.H. Mohr, G.M. Mudd, D. Giurco, Lithium resources and production: critical assessment and global projections. Minerals 2(3), 65–84 (2012)
Neupane, G. and Wendt, D.S., February. Assessment of mineral resources in geothermal brines in the US. In Proceedings of the 42nd Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, CA, USA, pp. 13–15 (2017)
F.S.T. Haklıdır, T.O. Balaban, A review of mineral precipitation and effective scale inhibition methods at geothermal power plants in West Anatolia (Turkey). Geothermics 80, 103–118 (2019). https://doi.org/10.1016/j.geothermics.2019.02.013
Schultze, L.E. and Bauer, D.J., Recovering lithium chloride from a geothermal brine. Report of investigations/1984 (No. PB-84–224500; BM-RI-8883). Bureau of Mines, Reno, NV (USA). Reno Research Center, (1984)
H. Wang, Y. Zhong, B. Du, Y. Zhao, M. Wang, Recovery of both magnesium and lithium from high Mg/Li ratio brines using a novel process. Hydrometallurgy 175, 102–108 (2018)
T.Y. Huang, J.R. Perez-Cardona, F. Zhao, J.W. Sutherland, M.P. Paranthaman, Life cycle assessment and techno-economic assessment of lithium recovery from geothermal brine. ACS Sustain. Chem. Eng. 9(19), 6551–6560 (2021)
G. Yan, G. Kim, R. Yuan, E. Hoenig, F. Shi, W. Chen, Y. Han, Q. Chen, J.-M. Zuo, W. Chen, C. Liu, The role of solid solutions in iron phosphate-based electrodes for selective electrochemical lithium extraction. Nat. Commun. 13(1), 4579 (2022)
Bourcier, W.L., Lin, M. and Nix, G., Recovery of minerals and metals from geothermal fluids (No. UCRL-CONF-215135). Lawrence Livermore National Lab. (LLNL), Livermore, CA (United States). Recovery of minerals and metals from geothermal fluids (No. UCRL-CONF-215135). Lawrence Livermore National Lab. (LLNL), Livermore, CA (United States), (2005)
N. Yasri, J. Hu, M.G. Kibria, E.P. Roberts, Electrocoagulation separation processes, in Multidisciplinary Advances in Efficient Separation Processes. ed. by I. Chernyshova, S. Ponnurangam, Q. Liu (American Chemical Society, Washington, 2020), pp.167–203
D.S. Patil, S.M. Chavan, J.U.K. Oubagaranadin, A review of technologies for manganese removal from wastewaters. J. Environ. Chem. Eng. 4(1), 468–487 (2016)
Z.Z. Chowdhury, S.M. Zain, A.K. Rashid, R.F. Rafque, K. Khalid, Breakthrough curve analysis for column dynamics sorption of Mn(II) ions from wastewater by using Mangostanagarcinia peel-based granular-activated carbon. J. Chem. 2013, 1–9 (2013). https://doi.org/10.1155/2013/959761
B.K. Pramanik, L.D. Nghiem, F.I. Hai, Extraction of strategically important elements from brines: constraints and opportunities. Water Res. 168, 115149 (2020)
Bujakowski, W., Tomaszewska, B. and Bodzek, M., Geothermal water treatment—preliminary experiences from Poland with a global overview of membrane and hybrid desalination technologies. Renewable Energy Applications for Freshwater Production (Sustainable Energy Developments) (red.: JochenBundschuh, Jan Hoinkis). Renewable Energy Applications for Freshwater Production (Sustainable Energy Developments), Taylor & Francis Ltd. CRC Press London, United Kingdom, p. 181–206 (2012)
Sulistiyono, E., Lalasari, L.H., Mayangsari, W. and Prasetyo, A.B., May. Study of lithium extraction from brine water, BledugKuwu, Indonesia by the precipitation series of oxalic acid and carbonate sodium. In AIP Conference Proceedings (1964, No. 1, p. 020007). AIP Publishing LLC, (2018)
E.B. Simsek, U. Beker, B.F. Senkal, Predicting the dynamics and performance of selective polymeric resins in a fixed bed system for boron removal. Desalination 349, 39–50 (2014)
Y.K. Recepoğlu, A. Yüksel, Cross-linked phosphorylated cellulose as a potential sorbent for lithium extraction from water: dynamic column studies and modeling. ACS Omega 7(43), 38957–38968 (2022)
T.E. Kose, N. Ozturk, Boron removal from aqueous solutions by ion-exchange resin: column sorption–elution studies. J. Hazard. Mater. 152(2), 744–749 (2008). https://doi.org/10.1016/j.jhazmat.2007.07.041
J. Liu, Y. Yan, H. Zhang, Adsorption dynamics of toluene in composite bed with microfibrous entrapped activated carbon. Chem. Eng. J. 173(2), 456–462 (2011). https://doi.org/10.1016/j.cej.2011.08.004
A.E. Yılmaz, R. Boncukcuoglu, M.T. Yılmaz, M.M. Kocakerim, Adsorption of boron from boron-containing wastewaters by ion exchange in a continuous reactor. J. Hazard. Mater. 117(2–3), 221–226 (2005)
H. Patel, Fixed-bed column adsorption study: a comprehensive review. Appl. Water Sci. 9(3), 45 (2019)
R. Kumari, S. Dey, A breakthrough column study for removal of malachite green using coco-peat. Int. J. Phytorem. 21(12), 1263–1271 (2019)
E.I. Unuabonah, M.I. El-Khaiary, B.I. Olu-Owolabi, K.O. Adebowale, Predicting the dynamics and performance of a polymer–clay based composite in a fixed bed system for the removal of lead (II) ion. Chem. Eng. Res. Des. 90(8), 1105–1115 (2012)
Z.H. Du, M.C. Jia, J.F. Men, Removal of cesium from aqueous solution using PAN-based ferrocyanide composite spheres: adsorption on a fixed-bed column. Appl. Mech. Mater. 496, 259–263 (2014). https://doi.org/10.4028/www.scientific.net/AMM.496-500.259
P.D. Saha, P. Bhattacharya, K. Sinha, S. Chowdhury, Biosorption of Congo red and indigo carmine by nonviable biomass of a new Dietzia strain isolated from the effluent of a textile industry. Desalin. Water Treat. 51(28–30), 5840–5847 (2013)
V. Mishra, C. Balomajumder, V.K. Agarwal, Zn (II) ion biosorption onto surface of eucalyptus leaf biomass: isotherm, kinetic, and mechanistic modeling. Clean-Soil, Air, Water 38(11), 1062–1073 (2010)
H. Wang, X. Yuan, Z. Wu, L. Wang, X. Peng, L. Leng, G. Zeng, Removal of basic dye from aqueous solution using Cinnamomumcamphora sawdust: kinetics, isotherms, thermodynamics, and mass-transfer processes. Sep. Sci. Technol. 49(17), 2689–2699 (2014)
R. Kumari, M.A. Khan, M. Mahto, M.A. Qaiyum, J. Mohanta, B. Dey, S. Dey, Dewaxed honeycomb as an economic and sustainable scavenger for malachite green from water. ACS Omega 5(31), 19548–19556 (2020). https://doi.org/10.1021/acsomega.0c02011
S.R. Manippady, A. Singh, B.M. Basavaraja, A.K. Samal, S. Srivastava, M. Saxena, Iron–carbon hybrid magnetic nanosheets for adsorption-removal of organic dyes and 4-nitrophenol from aqueous solution. ACS Appl. Nano Mater. 3(2), 1571–1582 (2020)
X. Han, J. Yuan, X. Ma, Adsorption of malachite green from aqueous solutions onto lotus leaf equilibrium kinetic and thermodynamic studies. Desalin. water Treat. 52(28–30), 5563–5574 (2014). https://doi.org/10.1080/19443994.2013.813102
S. Rangabhashiyam, S. Lata, P. Balasubramanian, Biosorption characteristics of methylene blue and malachite green from simulated wastewater onto Carica papaya wood biosorbent. Surf. Interfaces 10, 197–215 (2018)
R. Mosbah, M. Sahmoune, Biosorption of heavy metals by Streptomyces species—an overview. Open Chem. 11(9), 1412–1422 (2013)
P.H. Pacheco, R.A. Gil, S.E. Cerutti, P. Smichowski, L.D. Martinez, Biosorption: a new rise for elemental solid phase extraction methods. Talanta 85(5), 2290–2300 (2011)
M.A. Shaker, Thermodynamic profile of some heavy metal ions adsorption onto biomaterial surfaces. Am. J. Appl. Sci 4(8), 605–612 (2007)
Demir, M.M., Toprak, S., Oncel, C., Yilmaz, S., BABA, A. and AksoyKoc, G., Lithium Extraction from Geothermal Brine Using Γ-Mno2: A Case Study for Tuzla Geothermal Power Plant. Available at SSRN 4239318 (2022)
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no potential conflict of interest with respect to research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pawar, R., Santara, S., Sircar, A. et al. Extraction of salt and base metals from geothermal water: Kinetic modeling and mechanism. MRS Energy & Sustainability 10, 219–237 (2023). https://doi.org/10.1557/s43581-023-00066-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43581-023-00066-y