Abstract
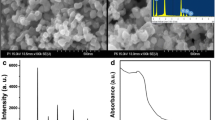
The nanorods of all-inorganic lead-free perovskite cesium titanium bromide (CsTiBr3) are synthesized by solvothermal method for the first time. The thermogravimetric analysis reveals that there are no phase transition and crystallization processes for a temperature range of 25–700°C. The CsTiBr3 perovskite nanorods exhibit excellent photocatalytic degradation of congo red (CR) dye. The CR dye has complete degradation within 60 min under direct sunlight exposure using 5 mg of CsTiBr3 as a catalyst. The XRD analysis of the catalyst after photocatalytic degradation ensures the reusability of CsTiBr3.The complete removal of organic components of the dye is confirmed by COD analysis.
Graphical abstract





Similar content being viewed by others
Data availability
Authors are ready to make all data available on reasonable request.
References
M.A.J. Kouhbanani, N. Beheshtkhoo, S. Taghizadeh, A.M. Amani, V. Alimardani, One-step green synthesis and characterization of iron oxide nanoparticles using aqueous leaf extract of Teucrium polium and their catalytic application in dye degradation. Adv. Nat. Sci.: Nanosci. Nanotechnol. 10, 015007 (2019)
D.O. Oyeniran, T.O. Sogbanmu, T.A. Adesalu, Antibiotics, algal evaluations and subacute effects of abattoir wastewater on liver function enzymes, genetic and haematologic biomarkers in the freshwater fish, Clarias gariepinus. Ecotoxicol. Environ. Saf. 1(212), 111982 (2021)
L. Ayed, A. Mahdhi, A. Cheref, A. Bakhrouf, Decolorization and degradation of azo dye methyl red by an isolated Sphingomonas paucimobilis: biotoxicity and metabolites characterization. Desalination 274(1–3), 272–277 (2011)
A. Kumar, A. Kumar, V. Krishnan, Perovskite oxide based materials for energy and environment-oriented photocatalysis. ACS Catal. 10(17), 10253–10315 (2020)
M. Kulbak, S. Gupta, N. Kedem, I. Levine, T. Bendikov, G. Hodes, D. Cahen, Cesium enhances long-term stability of lead bromide perovskite-based solar cells. J. Phys. Chem. Lett. 7(1), 167–172 (2016)
W.F. Zhang, J. Tang, J. Ye, Photoluminescence and photocatalytic properties of SrSnO3 perovskite. Chem. Phys. Lett. 418(1–3), 174–178 (2006)
J.A. Mares, C. Pedrini, B. Moine, K. Blazek, J. Kvapil, Optical studies of Ce3+-doped gadolinium aluminium perovskite single crystals. Chem. Phys. Lett. 206(1–4), 9–14 (1993)
Z. Zhang, Z. Chen, J. Zhang, W. Chen, J. Yang, X. Wen, B. Wang, N. Kobamoto, L. Yuan, J.A. Stride, G.J. Conibeer, Significant improvement in the performance of PbSe quantum dot solar cell by introducing a CsPbBr3 perovskite colloidal nanocrystal back layer. Adv. Energy Mater. 7(5), 1601773 (2017)
M. Saliba, T. Matsui, K. Domanski, J.Y. Seo, A. Ummadisingu, S.M. Zakeeruddin, J.P. Correa-Baena, W.R. Tress, A. Abate, A. Hagfeldt, M. Grätzel, Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 354(6309), 206–209 (2016)
I. Chung, B. Lee, J. He, R.P. Chang, M.G. Kanatzidis, All-solid-state dye-sensitized solar cells with high efficiency. Nature 485(7399), 486–489 (2012)
J. Liang, C. Wang, Y. Wang, Z. Xu, Z. Lu, Y. Ma, H. Zhu, Y. Hu, C. Xiao, X. Yi, G. Zhu, All-inorganic perovskite solar cells. J. Am. Chem. Soc. 138(49), 15829–15832 (2016)
S. Shrestha, X. Li, H. Tsai, C.H. Hou, H.H. Huang, D. Ghosh, J.J. Shyue, L. Wang, S. Tretiak, X. Ma, W. Nie, Long carrier diffusion length in two-dimensional lead halide perovskite single crystals. Chem 8(4), 1107–1120 (2022)
H. Fu, Review of lead-free halide perovskites as light-absorbers for photovoltaic applications: from materials to solar cells. Sol. Energy Mater. Sol. Cells 1(193), 107–132 (2019)
Q. Van Le, H.W. Jang, S.Y. Kim, Recent advances toward high-efficiency halide perovskite light-emitting diodes: review and perspective. Small Methods 2(10), 1700419 (2018)
D. Yang, J. Lv, X. Zhao, Q. Xu, Y. Fu, Y. Zhan, A. Zunger, L. Zhang, Functionality-directed screening of Pb-free hybrid organic–inorganic perovskites with desired intrinsic photovoltaic functionalities. Chem. Mater. 29(2), 524–538 (2017)
X.G. Zhao, J.H. Yang, Y. Fu, D. Yang, Q. Xu, L. Yu, S.H. Wei, L. Zhang, Design of lead-free inorganic halide perovskites for solar cells via cation-transmutation. J. Am. Chem. Soc. 139(7), 2630–2638 (2017)
M.G. Ju, M. Chen, Y. Zhou, H.F. Garces, J. Dai, L. Ma, N.P. Padture, X.C. Zeng, Earth-abundant nontoxic titanium (IV)-based vacancy-ordered double perovskite halides with tunable 1.0 to 1.8 eV bandgaps for photovoltaic applications. ACS Energy Lett. 3(2), 297–304 (2018)
J. Zhu, P.Z. Li, W. Guo, Y. Zhao, R. Zou, Titanium-based metal–organic frameworks for photocatalytic applications. Coord. Chem. Rev. 359, 80–101 (2018)
M. Devi, M.R. Panigrahi, Effect of Mn doping on the optical and electrical properties of TiO2 thin film prepared by unconventional sol–gel route. IOP Conf. Ser.: Mater. Sci. Eng. 653(1), 012018 (2019)
M.R. Panigrahi, M. Devi, Variation of optical and electrical properties of Zr doped TiO2 thin films with different annealing temperatures. J. Phys.: Conf. Ser. 1172(1), 012046 (2019)
M. Devi, M.R. Panigrahi, Effect of annealing temperature on the optical and electrical properties of Mg doped TiO2 thin films. Exp. Theor. Nanotechnol. 1, 69–79 (2017)
A.K. Sahoo, M.R. Panigrahi, Optical characterization of BMO thin film prepared by an unconventional sol-gel method. J. Sol–Gel Sci. Technol. 103(2), 565–575 (2022)
M. Devi, M.R. Panigrahi, U.P. Singh, Synthesis of TiO2 nanocrystalline powder prepared by sol-gel technique using TiO2 powder reagent. Adv. Appl. Sci. Res 5(3), 140–145 (2014)
M. Chen, M.G. Ju, A.D. Carl, Y. Zong, R.L. Grimm, J. Gu, X.C. Zeng, Y. Zhou, N.P. Padture, Cesium titanium (IV) bromide thin films based stable lead-free perovskite solar cells. Joule 2(3), 558–570 (2018)
K.B. Beegum, S. Sasi, A. Mathew, A.S. Asha, R. Reshmi, Nano fibers of lead free perovskite cesium titanium bromide (CsTiBr 3) thin films by in-house deposition technique. Phys. Scr. 96(5), 055707 (2021)
D. Kong, D. Cheng, X. Wang, K. Zhang, H. Wang, K. Liu, H. Li, X. Sheng, L. Yin, Solution processed lead-free cesium titanium halide perovskites and their structural, thermal and optical characteristics. J. Mater. Chem. C 8(5), 1591–1597 (2020)
G.K. Grandhi, A. Matuhina, M. Liu, S. Annurakshita, H. Ali-Löytty, G. Bautista, P. Vivo, Lead-free cesium titanium bromide double perovskite nanocrystals. Nanomaterials 11(6), 1458 (2021)
R.I. Walton, Subcritical solvothermal synthesis of condensed inorganic materials. Chem. Soc. Rev. 31(4), 230–238 (2002)
Joshi SS. Solar Induced CO 2 Reduction Achieved by Halide Tuning in Cesium Titanium (IV) Mixed Perovskite. In 2021 IEEE 21st International Conference on Nanotechnology (NANO) 2021 Jul 28 (pp. 299–302). IEEE.
D. Cardenas-Morcoso, A.F. Gualdrón-Reyes, A.B. Ferreira Vitoreti, M. García-Tecedor, S.J. Yoon, M. de la SolisFuente, I. Mora-Seró, S. Gimenez, Photocatalytic and photoelectrochemical degradation of organic compounds with all-inorganic metal halide perovskite quantum dots. J. Phys. Chem. Lett. 10(3), 630–636 (2019)
S. Niu, J. Milam-Guerrero, Y. Zhou, K. Ye, B. Zhao, B.C. Melot, J. Ravichandran, Thermal stability study of transition metal perovskite sulfides. J. Mater. Res. 33, 4135–4143 (2018)
A. Moquim, M.R. Panigrahi, Phase transition and relaxor nature of (Ba 0.77 Ca 0.23)(Ti 0.98 La 0.02) O 3 ceramic prepared by mixed oxide route. J. Mater. Sci.: Mater. Electron. 26, 4956–4962 (2015)
M.R. Panigrahi, S. Panigrahi, Phase transition and dielectric study in Ba 0.95 Dy 0.05 TiO3 ceramic. Bull. Mater. Sci. 34, 927–931 (2011)
J.R. Chen, W.L. Wang, J.B. Li, G.H. Rao, X-ray diffraction analysis and specific heat capacity of (Bi1− xLax) FeO3 perovskites. J. Alloy. Compd. 459(1–2), 66–70 (2008)
S.S. Abdullahi, S. Güner, Y.K. Musa, B.I. Adamu, M.I. Abdulhamid, Sımple method for the determınatıon of band gap of a nanopowdered sample usıng Kubelka Munk theory. NAMP J. 35, 241–246 (2016)
S.K. Mohamed, S.H. Hegazy, N.A. Abdelwahab, A.M. Ramadan, Coupled adsorption-photocatalytic degradation of crystal violet under sunlight using chemically synthesized grafted sodium alginate/ZnO/Graphene oxide composite. Int. J. Biol. Macromol. 1(108), 1185–1198 (2018)
K.A. Huynh, D.L. Nguyen, V.H. Nguyen, D.V. Vo, Q.T. Trinh, T.P. Nguyen, S.Y. Kim, Q.V. Le, Halide perovskite photocatalysis: progress and perspectives. J. Chem. Technol. Biotechnol. 95(10), 2579–2596 (2020)
M. Thomas, G.A. Naikoo, M.U. Sheikh, M. Bano, F. Khan, Effective photocatalytic degradation of Congo red dye using alginate/carboxymethyl cellulose/TiO2 nanocomposite hydrogel under direct sunlight irradiation. J. Photochem. Photobiol. A 15(327), 33–43 (2016)
Acknowledgments
The authors acknowledge DST-SERB(CRG/2018/003785) for the financial support, STIC and Department of Physics-CUSAT, CMET Thrissur, and Union Christian College-Aluva for the characterizations provided.
Funding
This work was funded by DST-SERB, CRG/2018/003785.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Beegum, K.A.B., Thomas, C., Sasi, S. et al. Degradation of congo red dye by thermally stable lead-free cesium titanium bromide (CsTiBr3) perovskite nanorods. MRS Communications 13, 1281–1287 (2023). https://doi.org/10.1557/s43579-023-00452-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43579-023-00452-0