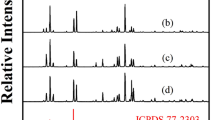
Abstract
Struvite or magnesium ammonium phosphate hexahydrate (H16MgNO10P) was synthesized in the presence of sodium alginate to determine the impact of extracellular polymeric substances (EPS) on struvite formation. Bulk crystallization of struvite at a 1.3:1:1 molar ratio of Mg:N:P, respectively, showed that in the presence of increasing sodium alginate concentration, struvite crystal habit changed from prismatic to plate-like twinned morphology. A possible mechanism of the adsorption of sodium alginate at [001]cc facets of struvite is proposed.
Graphical abstract





Similar content being viewed by others
References
L. Wei et al., Probing the effect of humic acid on the nucleation and growth kinetics of struvite by constant composition technique. Chem. Eng. J. 378, 122130 (2019). https://doi.org/10.1016/j.cej.2019.122130
Md. Mukhlesur Rahman, M.A. Mohd, U.R. Salleh, A. Ahsan, M.M. Hossain, C.S. Ra, Production of slow release crystal fertilizer from wastewaters through struvite crystallization—A review. Arab. J. Chem. 7, 139–155 (2014). https://doi.org/10.1016/j.arabjc.2013.10.007
R. Taddeo, M. Honkanen, K. Kolppo, R. Lepistö, Nutrient management via struvite precipitation and recovery from various agroindustrial wastewaters: process feasibility and struvite quality. J. Environ. Manage. 212, 433–439 (2018). https://doi.org/10.1016/j.jenvman.2018.02.027
A. Muhmood, S. Wu, J. Lu, Z. Ajmal, H. Luo, R. Dong, Nutrient recovery from anaerobically digested chicken slurry via struvite: Performance optimization and interactions with heavy metals and pathogens. Sci. Total Environ. 635, 1–9 (2018). https://doi.org/10.1016/j.scitotenv.2018.04.129
H. Wu, C. Vaneeckhaute, Nutrient recovery from wastewater: a review on the integrated physicochemical technologies of ammonia stripping, adsorption and struvite precipitation. Chem. Eng. J. 433, 133664 (2022). https://doi.org/10.1016/j.cej.2021.133664
J. Monetti, P. Ledezma, B. Virdis, S. Freguia, Nutrient recovery by bio-electroconcentration is limited by wastewater conductivity. ACS Omega 4, 2152–2159 (2019). https://doi.org/10.1021/acsomega.8b02737
A. Muhmood, J. Lu, R. Dong, S. Wu, Formation of struvite from agricultural wastewaters and its reuse on farmlands: status and hindrances to closing the nutrient loop. J. Environ. Manage. 230, 1–13 (2019). https://doi.org/10.1016/j.jenvman.2018.09.030
C. Zhang, D. Hu, R. Yang, Z. Liu, Effect of sodium alginate on phosphorus recovery by vivianite precipitation. J. Environ. Sci. 93, 164–169 (2020). https://doi.org/10.1016/j.jes.2020.04.007
Y. Luo et al., Bacterial mineralization of struvite using MgO as magnesium source and its potential for nutrient recovery. Chem. Eng. J. 351, 195–202 (2018). https://doi.org/10.1016/j.cej.2018.06.106
N.Y. Acelas, E. Flórez, D. López, Phosphorus recovery through struvite precipitation from wastewater: effect of the competitive ions. Desalin. Water Treat. 54(9), 2468–2479 (2015). https://doi.org/10.1080/19443994.2014.902337
B. Liu, A. Giannis, J. Zhang, V.W.-C. Chang, J.-Y. Wang, Characterization of induced struvite formation from source-separated urine using seawater and brine as magnesium sources. Chemosphere 93(11), 2738–2747 (2013). https://doi.org/10.1016/j.chemosphere.2013.09.025
N. Hutnik, K. Piotrowski, B. Wierzbowska, A. Matynia, Continuous reaction crystallization of struvite from phosphate(V) solutions containing calcium ions. Cryst. Res. Technol. 46(5), 443–449 (2011). https://doi.org/10.1002/crat.201100049
A. Siciliano, C. Limonti, G.M. Curcio, R. Molinari, Advances in struvite precipitation technologies for nutrients removal and recovery from aqueous waste and wastewater. Sustainability (2020). https://doi.org/10.3390/su12187538
C. Moragaspitiya, J. Rajapakse, G.J. Millar, Effect of struvite and organic acids on immobilization of copper and zinc in contaminated bio-retention filter media. J. Environ. Sci. 97, 35–44 (2020). https://doi.org/10.1016/j.jes.2020.04.023
D.S. Perwitasari, S. Muryanto, J. Jamari, A.P. Bayuseno, Optimization of struvite crystallization and heavy metal recovery in wastewater using response surface methodology. Orient. J. Chem. 34(1), 336–345 (2018). https://doi.org/10.13005/ojc/340136
A. Capdevielle, E. Sýkorová, F. Béline, M.-L. Daumer, Effects of organic matter on crystallization of struvite in biologically treated swine wastewater. Environ. Technol. 37(7), 880–892 (2016). https://doi.org/10.1080/09593330.2015.1088580
J. Wu et al., Effects of physicochemical parameters on struvite crystallization based on kinetics. Int. J. Environ. Res. Public Health (2022). https://doi.org/10.3390/ijerph19127204
A.N. Kofina, K.D. Demadis, P.G. Koutsoukos, The effect of citrate and phosphocitrate on struvite spontaneous precipitation. Cryst. Growth Des. 7(12), 2705–2712 (2007). https://doi.org/10.1021/cg0603927
D. Kim, J. Moore, C.P. McCoy, N.J. Irwin, J.D. Rimer, Engaging a battle on two fronts: dual role of polyphosphates as potent inhibitors of struvite nucleation and crystal growth. Chem. Mater. 32(19), 8672–8682 (2020). https://doi.org/10.1021/acs.chemmater.0c03180
Q. Zhang, S. Zhao, X. Ye, W. Xiao, Effects of organic substances on struvite crystallization and recovery. Desalin. Water Treat. 57(23), 10924–10933 (2016). https://doi.org/10.1080/19443994.2015.1040850
A. Rabinovich, A.A. Rouff, Effect of phenolic organics on the precipitation of struvite from simulated dairy wastewater. ACS ES&T Water 1(4), 910–918 (2021). https://doi.org/10.1021/acsestwater.0c00234
L.Z. Lakshtanov, D.A. Belova, D.V. Okhrimenko, S.L.S. Stipp, Role of alginate in calcite recrystallization. Cryst. Growth Des. 15(1), 419–427 (2015). https://doi.org/10.1021/cg501492c
E. Dalas, K. Barlos, D. Gatos, P. Manis, Effect of the cysteine-rich Mdm2 peptide in the crystal growth of hydroxyapatite in aqueous solution. Cryst. Growth Des. 7(1), 132–135 (2007). https://doi.org/10.1021/cg0506121
L. Wei, T. Hong, H. Liu, T. Chen, The effect of sodium alginate on struvite crystallization in aqueous solution: a kinetics study. J. Cryst. Growth 473, 60–65 (2017). https://doi.org/10.1016/j.jcrysgro.2017.03.039
I.J.C. Dela Cruz, J.V. Perez, B.G. Alamani, G. Capellades, A.S. Myerson, Influence of volume on the nucleation of model organic molecular crystals through an induction time approach. Cryst. Growth Des. (2021). https://doi.org/10.1021/acs.cgd.1c00101
I. Rodríguez-Ruiz et al., Transient calcium carbonate hexahydrate (ikaite) nucleated and stabilized in confined nano- and picovolumes. Cryst. Growth Des. 14(2), 792–802 (2014). https://doi.org/10.1021/cg401672v
H. Arslanoglu, Adsorption of micronutrient metal ion onto struvite to prepare slow release multielement fertilizer: copper(II) doped-struvite. Chemosphere 217, 393–401 (2019). https://doi.org/10.1016/j.chemosphere.2018.10.207
E. Heraldy, F. Rahmawati, D.P. Putra, Preparation of struvite from desalination waste. J. Environ. Chem. Eng. 5(1666), 1675 (2017). https://doi.org/10.1016/j.jece.2017.03.005
K. Zhao et al., Adsorption and photocatalytic degradation of methyl orange imprinted composite membranes using TiO2/calcium alginate hydrogel as matrix. Catal. Today. 236, 127–134 (2014). https://doi.org/10.1016/j.cattod.2014.03.041
Z. Ye, Y. Shen, X. Ye, Z. Zhang, S. Chen, J. Shi, Phosphorus recovery from wastewater by struvite crystallization: property of aggregates. J. Environ. Sci. 26(5), 991–1000 (2014). https://doi.org/10.1016/S1001-0742(13)60536-7
Acknowledgments
This research work acknowledges the Department of Science Engineering Research and Development for Technology (DOST-ERDT) for the financial assistance. It also acknowledges the Chemical Engineering Analytical Laboratory (CEAL), Department of Chemical Engineering, University of the Philippines Diliman, Quezon City, for the SEM, FTIR and IC training. The Crystallization and Bio-Inspired Engineering Group (CBEG) under the Bioprocess Engineering Laboratory is acknowledged for technical support and for the equipment used during the experiment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cruz, I.J.C.D., Potato, D.N.C. & Alamani, B.G. On the influence of sodium alginate on struvite crystal morphology. MRS Communications 13, 641–646 (2023). https://doi.org/10.1557/s43579-023-00406-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43579-023-00406-6