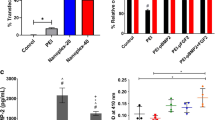
Abstract
Dental pulpitis, a common dental disease, poses a significant challenge for modern dentistry because of the intricate framework and limited regenerative capacity of dental pulp. Traditional pulp revascularization techniques have limitations in tissue regeneration and control over the type of regenerated tissue. The objective of this investigation is to investigate the capability of an innovative nanofiber tissue engineering scaffold for the regeneration of dental pulp, thereby delving into its latent possibilities. The scaffold, composed of polylactic acid-glycolic acid copolymer (PLGA) and dimethyloxalylglycine-Mesoporous silica nanoparticles (DMOG@MSNs), was fabricated using electrospinning technology. The DMOG@MSNs-PLGA scaffold exhibited good hydrophilicity, biocompatibility, and prolonged liberation of DMOG. In vitro assays demonstrated the scaffold promoted the proliferation, migration, and multidirectional differentiation of stem cells from apical papilla. The results indicate that DMOG@MSNs-PLGA scaffold holds promise for dental pulp regeneration, offering a potential strategy for regenerative endodontic therapy.
Trial registration number: JNSKQYY-2022-001, date of registration: March 9, 2022.
Graphical abstract







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
N. Raslan, W.-E. Wetzel, Exposed human pulp caused by trauma and/or caries in primary dentition: a histological evaluation. Dent. Traumatol.Traumatol. 22(3), 145 (2006). https://doi.org/10.1111/j.1600-9657.2006.00410.x
L. Hu, Y. Liu, S. Wang, Stem cell-based tooth and periodontal regeneration. Oral Dis. 24(5), 696 (2018). https://doi.org/10.1111/odi.12703
G.T.-J. Huang, F. Garcia-Godoy, Missing concepts in De Novo pulp regeneration. J. Dent. Res. 93(8), 717 (2014). https://doi.org/10.1177/0022034514537829
G. Schmalz, M. Widbiller, K.M. Galler, Clinical perspectives of pulp regeneration. J. Endod.Endod. 46(9S), S161 (2020). https://doi.org/10.1016/j.joen.2020.06.037
Y. Wei, P. Lyu, R. Bi, X. Chen, Y. Yu, Z. Li, Y. Fan, Neural regeneration in regenerative endodontic treatment: an overview and current trends. Int. J. Mol. Sci. 23(24), 15492 (2022). https://doi.org/10.3390/ijms232415492
D. Ricucci, S. Loghin, J.F. Siqueira, Correlation between clinical and histologic pulp diagnoses. J. Endod.Endod. 40(12), 1932 (2014). https://doi.org/10.1016/j.joen.2014.08.010
Z.C. Cehreli, G.E. Unverdi, E. Ballikaya, Deciduous tooth pulp autotransplantation for the regenerative endodontic treatment of permanent teeth with pulp necrosis: a case series. J. Endod.Endod. 48(5), 669 (2022). https://doi.org/10.1016/j.joen.2022.01.015
G.T.-J. Huang, S. Gronthos, S. Shi, Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J. Dent. Res. 88(9), 792 (2009). https://doi.org/10.1177/0022034509340867
Y. Li, Y. Lu, I. Maciejewska, K.M. Galler, A. Cavender, R.N. D’Souza, TWIST1 promotes the odontoblast-like differentiation of dental stem cells. Adv. Dent. Res. (2011). https://doi.org/10.1177/0022034511405387
J. Yu, H. He, C. Tang, G. Zhang, Y. Li, R. Wang, J. Shi, Y. Jin, Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 11, 32 (2010). https://doi.org/10.1186/1471-2121-11-32
D.B. Sequeira, A.R. Oliveira, C.M. Seabra, P.J. Palma, C. Ramos, M.H. Figueiredo, A.C. Santos, A.L. Cardoso, J. Peça, J.M. Santos, Regeneration of pulp-dentin complex using human stem cells of the apical papilla: in vivo interaction with two bioactive materials. Clin. Oral Investig.. Oral Investig. 25(9), 5317 (2021). https://doi.org/10.1007/s00784-021-03840-9
J. Xie, X. Li, Y. Xia, Putting electrospun nanofibers to work for biomedical research. Macromol. Rapid Commun.. Rapid Commun. 29(22), 1775 (2008). https://doi.org/10.1002/marc.200800381
X. Lu, C. Wang, Y. Wei, One-dimensional composite nanomaterials: synthesis by electrospinning and their applications. Small 5(21), 2349 (2009). https://doi.org/10.1002/smll.200900445
W. Liu, S. Thomopoulos, Y. Xia, Electrospun nanofibers for regenerative medicine. Adv Healthc Mater 1(1), 10 (2012). https://doi.org/10.1002/adhm.201100021
X. Wang, B. Ding, B. Li, Biomimetic electrospun nanofibrous structures for tissue engineering. Mater Today (Kidlington) 16(6), 229 (2013). https://doi.org/10.1016/j.mattod.2013.06.005
S. Thakkar, M. Misra, Electrospun polymeric nanofibers: new horizons in drug delivery. Eur. J. Pharm. Sci. 107, 148 (2017). https://doi.org/10.1016/j.ejps.2017.07.001
L. Zhang, Y. Yu, K.-C. Feng, Y.-C. Chuang, X. Zuo, Y. Zhou, C.-C. Chang, M. Simon, M. Rafailovich, Templated dentin formation by dental pulp stem cells on banded collagen bundles nucleated on electrospun poly (4-vinyl pyridine) fibers in vitro. Acta Biomater. Biomater. 76, 80 (2018). https://doi.org/10.1016/j.actbio.2018.06.028
M.T.P. Albuquerque, M.C. Valera, M. Nakashima, J.E. Nör, M.C. Bottino, Tissue-engineering-based strategies for regenerative endodontics. J. Dent. Res. 93(12), 1222 (2014). https://doi.org/10.1177/0022034514549809
G. Chen, J. Chen, B. Yang, L. Li, X. Luo, X. Zhang, L. Feng, Z. Jiang, M. Yu, W. Guo, W. Tian, Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials 52, 56 (2015). https://doi.org/10.1016/j.biomaterials.2015.02.011
S.U. Rahman, S. Ponnusamy, M. Nagrath, P.R. Arany, Precision-engineered niche for directed differentiation of MSCs to lineage-restricted mineralized tissues. J. Tissue Eng. 13, 20417314211073936 (2022). https://doi.org/10.1177/20417314211073934
S. Türkkan, A.E. Pazarçeviren, D. Keskin, N.E. Machin, Ö. Duygulu, A. Tezcaner, Nanosized CaP-silk fibroin-PCL-PEG-PCL/PCL based bilayer membranes for guided bone regeneration. Mater. Sci. Eng. C 80, 484 (2017). https://doi.org/10.1016/j.msec.2017.06.016
E.A. Münchow, M.T.P. Albuquerque, B. Zero, K. Kamocki, E. Piva, R.L. Gregory, M.C. Bottino, Development and characterization of novel ZnO-loaded electrospun membranes for periodontal regeneration. Dent. Mater. 31(9), 1038 (2015). https://doi.org/10.1016/j.dental.2015.06.004
M.C. Bottino, K. Kamocki, G.H. Yassen, J.A. Platt, M.M. Vail, Y. Ehrlich, K.J. Spolnik, R.L. Gregory, Bioactive nanofibrous scaffolds for regenerative endodontics. J. Dent. Res. 92(11), 963 (2013). https://doi.org/10.1177/0022034513505770
G. Zanatta, M. Rudisile, M. Camassola, J. Wendorff, N. Nardi, C. Gottfried, P. Pranke, C.A. Netto, Mesenchymal stem cell adherence on poly(D, L-lactide-co-glycolide) nanofibers scaffold is integrin-beta 1 receptor dependent. J. Biomed. Nanotechnol.Nanotechnol. 8(2), 211 (2012). https://doi.org/10.1166/jbn.2012.1382
S. Jin, X. Xia, J. Huang, C. Yuan, Y. Zuo, Y. Li, J. Li, Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. Biomater. 127, 56 (2021). https://doi.org/10.1016/j.actbio.2021.03.067
M.J. Strowitzki, A.S. Ritter, G. Kimmer, M. Schneider, Hypoxia-adaptive pathways: a pharmacological target in fibrotic disease? Pharmacol. Res.. Res. 147, 104364 (2019). https://doi.org/10.1016/j.phrs.2019.104364
M.H.G. Costa, J. Serra, T.C. McDevitt, J.M.S. Cabral, C.L. da Silva, F.C. Ferreira, Dimethyloxalylglycine, a small molecule, synergistically increases the homing and angiogenic properties of human mesenchymal stromal cells when cultured as 3D spheroids. Biotechnol. J.. J. 16(5), e2000389 (2021). https://doi.org/10.1002/biot.202000389
F. Liu, X. Huang, Z. Luo, J. He, F. Haider, C. Song, L. Peng, T. Chen, B. Wu, Hypoxia-activated PI3K/Akt inhibits oxidative stress via the regulation of reactive oxygen species in human dental pulp cells. Oxid. Med. Cell. Longev.. Med. Cell. Longev. 2019, 6595189 (2019). https://doi.org/10.1155/2019/6595189
J. Zhou, C. Sun, SENP1/HIF-1α axis works in angiogenesis of human dental pulp stem cells. J. Biochem. Mol. Toxicol.Biochem. Mol. Toxicol. 34(3), e22436 (2020). https://doi.org/10.1002/jbt.22436
E. Shimizu, G. Jong, N. Partridge, P.A. Rosenberg, L.M. Lin, Histologic observation of a human immature permanent tooth with irreversible pulpitis after revascularization/regeneration procedure. J. Endod.Endod. 38(9), 1293 (2012). https://doi.org/10.1016/j.joen.2012.06.017
B.D. Smaila, S.D. Holland, F. Babaeijandaghi, H.G. Henderson, F.M.V. Rossi, M.S. Ramer, Systemic hypoxia mimicry enhances axonal regeneration and functional recovery following peripheral nerve injury. Exp. Neurol. 334, 113436 (2020). https://doi.org/10.1016/j.expneurol.2020.113436
Y. Li, W. Han, Y. Wu, K. Zhou, Z. Zheng, H. Wang, L. Xie, R. Li, K. Xu, Y. Liu, X. Wang, J. Xiao, Stabilization of hypoxia inducible factor-1α by dimethyloxalylglycine promotes recovery from acute spinal cord injury by inhibiting neural apoptosis and enhancing axon regeneration. J. NeurotraumaNeurotrauma 36(24), 3394 (2019). https://doi.org/10.1089/neu.2018.6364
S. Zippusch, K.F.W. Besecke, F. Helms, M. Klingenberg, A. Lyons, P. Behrens, A. Haverich, M. Wilhelmi, N. Ehlert, U. Böer, Chemically induced hypoxia by dimethyloxalylglycine (DMOG)-loaded nanoporous silica nanoparticles supports endothelial tube formation by sustained VEGF release from adipose tissue-derived stem cells. Regen. Biomater. 8(5), rbab039 (2021). https://doi.org/10.1093/rb/rbab039
Y. Zhu, F. Song, Y. Ju, L. Huang, L. Zhang, C. Tang, H. Yang, C. Huang, NAC-loaded electrospun scaffolding system with dual compartments for the osteogenesis of rBMSCs in vitro. Int. J. Nanomed.Nanomed. 14, 787 (2019). https://doi.org/10.2147/IJN.S183233
Z.-Q. Liu, L.-L. Shang, S.-H. Ge, Immunomodulatory effect of dimethyloxallyl glycine/nanosilicates-loaded fibrous structure on periodontal bone remodeling. J. Dent. Sci. 16(3), 937 (2021). https://doi.org/10.1016/j.jds.2020.10.008
X. Ren, Y. Han, J. Wang, Y. Jiang, Z. Yi, H. Xu, Q. Ke, An aligned porous electrospun fibrous membrane with controlled drug delivery—an efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. Biomater. 70, 140 (2018). https://doi.org/10.1016/j.actbio.2018.02.010
L. Shang, Z. Liu, B. Ma, J. Shao, B. Wang, C. Ma, S. Ge, Dimethyloxallyl glycine/nanosilicates-loaded osteogenic/angiogenic difunctional fibrous structure for functional periodontal tissue regeneration. Bioact. Mater. 6(4), 1175 (2021). https://doi.org/10.1016/j.bioactmat.2020.10.010
P.M. Amarasinghe, K.S. Katti, D.R. Katti, Nature of organic fluid—montmorillonite interactions: an FTIR spectroscopic study. J. Colloid Interface Sci. 337(1), 97 (2009). https://doi.org/10.1016/j.jcis.2009.05.011
X. Ji, H. Shao, X. Li, M.W. Ullah, G. Luo, Z. Xu, L. Ma, X. He, Z. Lei, Q. Li, X. Jiang, G. Yang, Y. Zhang, Injectable immunomodulation-based porous chitosan microspheres/HPCH hydrogel composites as a controlled drug delivery system for osteochondral regeneration. Biomaterials 285, 121530 (2022). https://doi.org/10.1016/j.biomaterials.2022.121530
L. He, J. Zhou, M. Chen, C.-S. Lin, S.G. Kim, Y. Zhou, L. Xiang, M. Xie, H. Bai, H. Yao, C. Shi, P.G. Coelho, T.G. Bromage, B. Hu, N. Tovar, L. Witek, J. Wu, K. Chen, W. Gu, J. Zheng, T.-J. Sheu, J. Zhong, J. Wen, Y. Niu, B. Cheng, Q. Gong, D.M. Owens, M. Stanislauskas, J. Pei, G. Chotkowski, S. Wang, G. Yang, D.J. Zegarelli, X. Shi, M. Finkel, W. Zhang, J. Li, J. Cheng, D.P. Tarnow, X. Zhou, Z. Wang, X. Jiang, A. Romanov, D.W. Rowe, S. Wang, L. Ye, J. Ling, J. Mao, Parenchymal and stromal tissue regeneration of tooth organ by pivotal signals reinstated in decellularized matrix. Nat. Mater. 18(6), 627 (2019). https://doi.org/10.1038/s41563-019-0368-6
S.N. Kaushik, B. Kim, A.M.C. Walma, S.C. Choi, H. Wu, J.J. Mao, H.-W. Jun, K. Cheon, Biomimetic microenvironments for regenerative endodontics. Biomater. Res. 20, 14 (2016). https://doi.org/10.1186/s40824-016-0061-7
E. Shimizu, D. Ricucci, J. Albert, A.S. Alobaid, J.L. Gibbs, G.T.-J. Huang, L.M. Lin, Clinical, radiographic, and histological observation of a human immature permanent tooth with chronic apical abscess after revitalization treatment. J. Endod.Endod. 39(8), 1078 (2013). https://doi.org/10.1016/j.joen.2013.04.032
A. Nosrat, A. Kolahdouzan, F. Hosseini, E.A. Mehrizi, P. Verma, M. Torabinejad, Histologic outcomes of uninfected human immature teeth treated with regenerative endodontics: 2 case reports. J. Endod.Endod. 41(10), 1725 (2015). https://doi.org/10.1016/j.joen.2015.05.004
M. Song, Y. Cao, S.-J. Shin, W.-J. Shon, N. Chugal, R.H. Kim, E. Kim, M.K. Kang, Revascularization-associated intracanal calcification: assessment of prevalence and contributing factors. J. Endod.Endod. 43(12), 2025 (2017). https://doi.org/10.1016/j.joen.2017.06.018
H. Chu, Y. Zhang, F. Wang, T. Feng, L. Wang, D. Wang, Effect of graphene oxide on mechanical properties and durability of ultra-high-performance concrete prepared from recycled sand. Nanomaterials (Basel) 10(9), 1718 (2020). https://doi.org/10.3390/nano10091718
N. Baishya, M. Mamouei, K. Budidha, M. Qassem, P. Vadgama, P.A. Kyriacou, Investigations into the effects of pH on quantitative measurements of lactate in biological media using ATR-FTIR spectroscopy. Molecules 25(16), 3695 (2020). https://doi.org/10.3390/molecules25163695
H. Wang, H. Yu, T.N. Tran, K. Fu, K. Kiley, S. Kullar, J. Hu, M. Kamberi, Chemical characterization of leachables in catheter device. ACS Omega 7(51), 48291 (2022). https://doi.org/10.1021/acsomega.2c06473
M. Ziąbka, E. Menaszek, J. Tarasiuk, S. Wroński, Biocompatible nanocomposite implant with silver nanoparticles for otology-in vivo evaluation. Nanomaterials (Basel) 8(10), 764 (2018). https://doi.org/10.3390/nano8100764
H. Song, Y. Zhang, Z. Zhang, S. Xiong, X. Ma, Y. Li, Hydroxyapatite/NELL-1 nanoparticles electrospun fibers for osteoinduction in bone tissue engineering application. Int. J. Nanomed.Nanomed. 16, 4321 (2021). https://doi.org/10.2147/IJN.S309567
Acknowledgments
The authors thank Peng Yan (College of Basic Medical Sciences, Binzhou Medical University) for providing an electrospinning machine.
Funding
This work was supported by the “Clinical + X” scientific and technological innovation project of Binzhou Medical University (BY2021LCX08); Natural Science Foundation of Shandong Province (ZR2019BH040); Ji Nan Science & Technology Bureau (202134005); Research Assistance Fund for the President of Ji Nan Stomatological Hospital.
Author information
Authors and Affiliations
Contributions
CL: methodology, formal analysis, writing-original draft. LL: investigation, formal analysis. AL: guidance of experimental techniques. XY: disinfection of experimental materials. SW: data curation. SX: data curation. XY: assistance of project administration, co-supervision, funding acquisition. LZ: funding acquisition, project administration, review & editing. YD: conceptualization, funding acquisition, project administration.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known conflicts of interest/competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Ethics approval was obtained from Jinan Stomatological Hospital for this study (Date of registration: March 9, 2022. Ethics approval number: JNSKQYY-2022-001).
Consent for publication
All authors agree to participate and agree to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Luan, L., Lyu, A. et al. Electrospun dimethyloxallylglycine sustained release scaffold for promoting the migration and multidirectional differentiation of stem cells from the apical papilla. Journal of Materials Research 39, 609–625 (2024). https://doi.org/10.1557/s43578-023-01253-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-023-01253-w