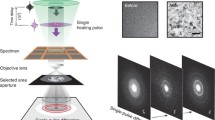
Abstract
The crystallization of an amorphous Ag–In–Sb–Te (AIST) phase change material (PCM) is studied using multiple in situ imaging techniques to directly quantify crystal growth rates over a broad range of temperatures. The measurable growth rates span from ≈ 10–9 to ≈ 20 m/s. Recent results using dynamic transmission electron microscopy (TEM), a photoemission TEM technique, and TEM with sub-framed imaging are reported here and placed into the context of previous growth rate measurements on AIST. Dynamic TEM experiments show a maximum observed crystal growth rate for as-deposited films to be > 20 m/s. It is shown that crystal growth above the glass transition can be imaged in a TEM through use of subframing and a high-frame-rate direct electron detection camera. Challenges associated with the determination of temperature during in situ TEM experiments are described. Preliminary nanocalorimetry results demonstrate the feasibility of collecting thermodynamic data for crystallization of PCMs with simultaneous TEM imaging.
Graphical abstract







Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
S.R. Ovshinsky, Reversible electrical switching phenomena in disordered structures. Phys. Rev. Lett. 21(20), 1450 (1968)
M. Chen, K.A. Rubin, R.W. Barton, Compound materials for reversible, phase-change optical-data storage. Appl. Phys. Lett. 49(9), 502 (1986)
Y.V. Pershin, M. Di Ventra, Memory effects in complex materials and nanoscale systems. Adv. Phys. 60(2), 145 (2011)
W. Zhang, R. Mazzarello, M. Wuttig, E. Ma, Designing crystallization in phase-change materials for universal memory and neuro-inspired computing. Nat. Rev. Mater. 4(3), 150 (2019)
Y.B. Li, Z.R. Wang, R. Midya, Q.F. Xia, J.J. Yang, Review of memristor devices in neuromorphic computing: materials sciences and device challenges. J. Phys. D-Appl. Phys. 51(50), 503002 (2018)
N. Yamada, E. Ohno, K. Nishiuchi, N. Akahira, M. Takao, Rapid-phase transitions of GeTe-Sb2Te3 pseudobinary amorphous thin-films for an optical disk memory. J. Appl. Phys. 69(5), 2849 (1991)
V. Weidenhof, N. Pirch, I. Friedrich, S. Ziegler, M. Wuttig, Minimum time for laser induced amorphization of Ge2Sb2Te5 films. J. Appl. Phys. 88(2), 657 (2000)
S.R. Elliott, Chalcogenide phase-change materials: past and future. Int. J. Appl. Glas. Sci. 6(1), 15 (2015)
J. Orava, A.L. Greer, Fast crystal growth in glass-forming liquids. J. Non-Cryst. Solids 451, 94 (2016)
M.M. Winseck, H.-Y. Cheng, G.H. Campbell, M.K. Santala, Crystallization kinetics of the phase change material GeSb6Te measured with dynamic transmission electron microscopy. Dalton Trans. 45, 9988 (2016)
J. Orava, A.L. Greer, D.W. Hewak, C.E. Smith, Characterization of supercooled liquid Ge2Sb2Te5 and its crystallization by ultrafast-heating calorimetry. Nat. Mater. 11, 279 (2012)
J. Orava, D.W. Hewak, A.L. Greer, Fragile-to-strong crossover in supercooled liquid Ag-In-Sb-Te studied by ultrafast calorimetry. Adv. Funct. Mater. 25(30), 4851 (2015)
J. Orava, A.L. Greer, Kissinger method applied to the crystallization of glass-forming liquids: regimes revealed by ultra-fast-heating calorimetry. Thermochim. Acta 603, 63 (2015)
B. Chen, J. Momand, P.A. Vermeulen, B.J. Kooi, Crystallization kinetics of supercooled liquid Ge-Sb based on ultrafast calorimetry. Cryst. Growth Des. 16(1), 242 (2016)
M. Salinga, E. Carria, A. Kaldenbach, M. Bornhoefft, J. Benke, J. Mayer, M. Wuttig, Measurement of crystal growth velocity in a melt-quenched phase-change material. Nat. Commun. 4, 1 (2013)
J. Kalb, F. Spaepen, M. Wuttig, Atomic force microscopy measurements of crystal nucleation and growth rates in thin films of amorphous Te alloys. Appl. Phys. Lett. 84(25), 5240 (2004)
V.L. Bird: Crystallization rates of AIST and Ge thin films by optical and electron microscopy, M.S. thesis, Materials Science (Oregon State University, Corvallis, 2019).
G. Eising, T. Van Damme, B.J. Kooi, Unraveling crystal growth in GeSb phase-change films in between the glass-transition and melting temperatures. Cryst. Growth Des. 14(7), 3392 (2014)
M.K. Santala, B.W. Reed, S. Raoux, T. Topuria, T. LaGrange, G.H. Campbell, Capturing irreversible reactions with nanosecond-scale dynamic TEM movies: measuring crystal growth rates during laser annealing of phase change materials. Microsc. Microanal. 19(S2), 1156 (2013)
B.W. Reed, A.A. Moghadam, R.S. Bloom, S.T. Park, A.M. Monterrosa, P.M. Price, C.M. Barr, S.A. Briggs, K. Hattar, J.T. McKeown, D.J. Masiel, Electrostatic subframing and compressive-sensing video in transmission electron microscopy. Struct. Dyn. 6(5), 54–303 (2019)
H. Iwasaki, M. Harigaya, O. Nonoyama, Y. Kageyama, M. Takahashi, K. Yamada, H. Deguchi, Y. Ide, Completely erasable phase-change optical disc. 2. Application of Ag-In-Sb-Te mixed-phase system for rewritable compact disc compatible with CD-velocity and double CD-velocity. Jpn. J. Appl. Phys. 32(11B), 5241 (1993)
H. Tashiro, M. Harigaya, Y. Kageyama, K. Ito, M. Shinotsuka, K. Tani, A. Watada, N. Yiwata, Y. Nakata, S. Emury, Structural analysis of Ag-In-Sb-Te phase-change material. Jpn. J. Appl. Phys. 41(6A), 3758 (2002)
T. Schroder, T. Rosenthal, C. Gold, E.W. Scheidt, W. Schnick, O. Oeckler, Two synthetic approaches to Ag3.4In3.7Sb76.4Te16.5 bulk samples and their transport properties. Zeitschrift Fur Anorganische Und Allgemeine Chemie 639(15), 2868 (2013)
T. Matsunaga, N. Yamada, Crystallographic studies on high-speed phase-change materials used for rewritable optical recording disks. Jpn. J. Appl. Phys. 43(7B), 4704 (2004)
D.J. Sarrach, J.P. DeNeufville, W.L. Haworth, Studies of amorphous Ge-Se-Te alloys (1): preparation and calorimetric observations. J. Non-Cryst. Solids 22(2), 245 (1976)
J.A. Kalb, M. Wuttig, F. Spaepen, Calorimetric measurements of structural relaxation and glass transition temperatures in sputtered films of amorphous Te alloys used for phase change recording. J. Mater. Res. 22(3), 748 (2007)
B.-S. Lee, R.M. Shelby, S. Raoux, C.T. Retter, G.W. Burr, S.N. Bogle, K. Darmawikarta, S.G. Bishop, J.R. Abelson, Nanoscale nuclei in phase change materials: origin of different crystallization mechanisms of Ge2Sb2Te5 and AgInSbTe. J. Appl. Phys. 115(6), 063506 (2014)
B.S. Lee, G.W. Burr, R.M. Shelby, S. Raoux, C.T. Rettner, S.N. Bogle, K. Darmawikarta, S.G. Bishop, J.R. Abelson, Observation of the role of subcritical nuclei in crystallization of a glassy solid. Science 326(5955), 980 (2009)
Y. Fukuyama, N. Yasuda, J. Kim, H. Murayama, Y. Tanaka, S. Kimura, K. Kato, S. Kohara, Y. Moritomo, T. Matsunaga, R. Kojima, N. Yamada, H. Tanaka, T. Ohshima, M. Takata, Time-resolved investigation of nanosecond crystal growth in rapid-phase-change materials: correlation with the recording speed of digital versatile disc media. Appl. Phys. Express. 1(4), 045001 (2008)
W.K. Njoroge, M. Wuttig, Crystallization kinetics of sputter-deposited amorphous AgInSbTe films. J. Appl. Phys. 90(8), 3816 (2001)
W.K. Njoroge, H. Dieker, M. Wuttig, Influence of dielectric capping layers on the crystallization kinetics of Ag5In6Sb59Te30 films. J. Appl. Phys. 96(5), 2624 (2004)
J. Orava, H. Weber, I. Kaban, A.L. Greer, Viscosity of liquid Ag-In-Sb-Te: evidence of a fragile-to-strong crossover. J. Chem. Phys. 144(19), 194503 (2016)
K.F. Kelton, A.L. Greer, Transient nucleation effects in glass-formation. J. Non-Cryst. Solids 79(3), 295 (1986)
S. Senkader, C.D. Wright, Models for phase-change of Ge2Sb2Te5 in optical and electrical memory devices. J. Appl. Phys. 95(2), 504 (2004)
C.A. Angell, Formation of glasses from liquids and biopolymers. Science 267(5206), 1924 (1995)
M.D. Ediger, P. Harrowell, L. Yu, Crystal growth kinetics exhibit a fragility-dependent decoupling from viscosity. J. Chem. Phys. 128(3), 034709 (2008)
C.A. Angell, Water-II is a strong liquid. J. Phys. Chem. 97(24), 6339 (1993)
S. Wei, P. Lucas, C.A. Angell, Phase change alloy viscosities down to Tg using Adam-Gibbs-equation fittings to excess entropy data: A fragile-to-strong transition. J. Appl. Phys. 118(3), 34–903 (2015)
S.L. Lai, G. Ramanath, L.H. Allen, P. Infante, Z. Ma, High-speed (104°C/s) scanning microcalorimetry with monolayer sensitivity (J/m2). Appl. Phys. Lett. 67(9), 1229 (1995)
M.D. Grapes, T. LaGrange, L.H. Friedman, B.W. Reed, G.H. Campbell, T.P. Weihs, D.A. LaVan, Combining nanocalorimetry and dynamic transmission electron microscopy for in situ characterization of materials processes under rapid heating and cooling. Rev. Sci. Instrum. 85(8), 084902 (2014)
M.D. Grapes, M.K. Santala, G.H. Campbell, D.A. LaVan, T.P. Weihs, A detailed study of the Al3Ni formation reaction using nanocalorimetry. Thermochim. Acta 658, 72 (2017)
M.R. Armstrong, K. Boyden, N.D. Browning, G.H. Campbell, J.D. Colvin, W.J. DeHope, A.M. Frank, D.J. Gibson, F. Hartemann, J.S. Kim, W.E. King, T.B. LaGrange, B.J. Pyke, B.W. Reed, R.M. Shuttlesworth, B.C. Stuart, B.R. Torralva, Practical considerations for high spatial and temporal resolution dynamic transmission electron microscopy. Ultramicroscopy 107(4–5), 356 (2007)
T. LaGrange, B.W. Reed, M.K. Santala, J.T. McKeown, A. Kulovits, J.M.K. Wiezorek, L. Nikolova, F. Rosei, B.J. Siwick, G.H. Campbell, Approaches for ultrafast imaging of transient materials processes in the transmission electron microscope. Micron 43(11), 1108 (2012)
G.H. Campbell, J.T. McKeown, M.K. Santala, Time resolved electron microscopy for in situ experiments. Appl. Phys. Rev. 1(4), 041101 (2014)
M.K. Santala, B.W. Reed, S. Raoux, T. Topuria, T. LaGrange, G.H. Campbell, Nanosecond-scale time-resolved electron imaging during laser crystallization of GeTe. Phys. Status Solidi B 249(10), 1907 (2012)
M.K. Santala, B.W. Reed, S. Raoux, T. Topuria, T. LaGrange, G.H. Campbell, Irreversible reactions studied with nanosecond TEM movies: laser crystallization of phase change materials. Appl. Phys. Lett. 102(17), 174–105 (2013)
M.K. Santala, B.W. Reed, T. Topuria, S. Raoux, S. Meister, Y. Cui, T. LaGrange, G.H. Campbell, N.D. Browning, Nanosecond in situ transmission electron microscope studies of the reversible Ge2Sb2Te5 crystalline ⇔ amorphous phase transformation. J. Appl. Phys. 111(2), 024309 (2012)
J.C. Mauro, Y.Z. Yue, A.J. Ellison, P.K. Gupta, D.C. Allan, Viscosity of glass-forming liquids. Proc. Natl. Acad. Sci. USA. 106(47), 19780 (2009)
B.L. Zink, F. Hellman, Specific heat and thermal conductivity of low-stress amorphous Si-N membranes. Solid State Commun. 129(3), 199 (2004)
F. Yi, L.H. Friedman, R. Chen, D.A. LaVan, Sample pattern and temperature distribution in nanocalorimetry measurements. J. Therm. Anal. Calorim. 138(5), 3367 (2019)
F. Yi, M.D. Grapes, D.A. LaVan, Practical guide to the design, fabrication, and calibration of NIST nanocalorimeters. J. Res. Natl. Inst. Std. Technol. 124, 3367 (2019)
F. Tabatabaei, G. Boussinot, R. Spatschek, E.A. Brener, M. Apel, Phase field modeling of rapid crystallization in the phase-change material AIST. J. Appl. Phys. 122(4), 045108 (2017)
T. Matsunaga, Y. Umetani, N. Yamada, Structural study of a Ag3.4In3.7Sb76.4Te16.5 quadruple compound utilized for phase-change optical disks. Phys. Rev. B. 64(18), 184116 (2001)
J. Akola, R.O. Jones, Structure of liquid phase change material AgInSbTe from density functional/molecular dynamics simulations. Appl. Phys. Lett. 94(25), 3 (2009)
J.A. Kalb, F. Spaepen, M. Wuttig, Kinetics of crystal nucleation in undercooled droplets of Sb- and Te-based alloys used for phase change recording. J. Appl. Phys. 98(5), 054910 (2005)
Acknowledgments
Effort by M.K.S. and I.M. was supported by the National Science Foundation, Division of Materials Research CER [Grant No. 1945520]. TEM was performed at the Oregon State University Electron Microscope Facility which is supported by NSF MRI Grant No. 1040588, the Murdock Charitable Trust, and the Oregon Nanoscience and Micro-Technologies Institute. M.K.S would like to thank Dr. John Roehling and Dr. Garth Egan for assistance with thin film deposition and DTEM experiments at LLNL. Portions of this work were performed under the auspices of the US Department of Energy by Lawrence Livermore National Laboratory. Lawrence Livermore National Laboratory is operated by Lawrence Livermore National Security, LLC., for the US Department of Energy, National Nuclear Security Administration under Contract No. DE-AC52-07NA27344. Work was performed at the Center for Integrated Nanotechnologies, a User Facility operated for the US DOE Office of Science at Sandia National Laboratories (SNL), managed and operated by National Technology & Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International, Inc., for the US DOE’s National Nuclear Security Administration under contract DE-NA-0003525. This work was performed under the Laboratory Directed Research and Development program at SNL. This material is based in part upon work supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Award No. DE-SC0013104. Views expressed here do not necessarily represent the views of the US DOE or US Government. Research was performed in part at the NIST Center for Nanoscale Science and Technology. Any mention of commercial products is for information only; it does not imply recommendation or endorsement by the NIST.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 18419 kb)
Supplementary file3 (MP4 19180 kb)
Supplementary file4 (MP4 3374 kb)
Supplementary file5 (MP4 3782 kb)
Rights and permissions
About this article
Cite this article
McGieson, I., Bird, V.L., Barr, C.M. et al. Crystallization kinetics and thermodynamics of an Ag–In–Sb–Te phase change material using complementary in situ microscopic techniques. Journal of Materials Research 37, 1281–1295 (2022). https://doi.org/10.1557/s43578-022-00486-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-022-00486-5