Abstract
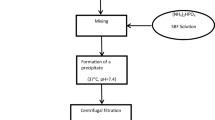
This work reports the development of hydroxyapatite-coated liposomes to encapsulate drug bupivacaine and quantum dots (CdSe) to be used as a theranostic tool. Systems like this allow monitoring drug distribution in vivo (because of the quantum dots) and they can modulate the rate of drug delivery by increasing drug concentration in situ (due to the hydroxyapatite coating). Liposomes were prepared by the injection technique, and their hydroxyapatite coating was obtained by co-precipitation of calcium and phosphate precursors. The increase in diameter and decrease in zeta potential of the hydroxyapatite-coated liposomes (LHAP) when compared to conventional liposomes evidenced that a core–shell was formed around the vesicles. X-ray diffraction and infrared spectroscopy measurements confirmed the presence of hydroxyapatite in the coating. The liposomes were then functionalized with CdSe to be used as theranostic and detected by photoluminescence. The bupivacaine was incorporated in liposomes/hydroxyapatite and its in vitro release kinetics was modulated, showing slower rates than those measured with the conventional liposomes.
Graphic abstract








Similar content being viewed by others
References
T. Matsumoto, M. Okazaki, M. Inoue, S. Yamaguchi, T. Kusunose, T. Toyonaga, Y. Hamada, J. Takahashi, Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials 25, 3807–3812 (2004). https://doi.org/10.1016/j.biomaterials.2003.10.081
E.C. Gaetti Jardim, P.L. dos Santos, J.F. Santiago Junior, E.G. Jardim Júnio, A.M. Aranega, I.R. Garcia Júnior, Enxerto ósseo em odontologia bone graft in odontology. Rev. Odontológica Araçatuba. 30, 24–28 (2009)
P. Kamalanathan, S. Ramesh, L.T. Bang, A. Niakan, C.Y. Tan, J. Purbolaksono, H. Chandran, W.D. Teng, Synthesis and sintering of hydroxyapatite derived from eggshells as a calcium precursor. Ceram. Int. 40, 16349–16359 (2014). https://doi.org/10.1016/j.ceramint.2014.07.074
A. Stoch, W. Jastrzȩbski, A. Brozek, J. Stoch, J. Szaraniec, B. Trybalska, G. Kmita, FTIR absorption-reflection study of biomimetic growth of phosphates on titanium implants. J. Mol. Struct. 555, 375–382 (2000). https://doi.org/10.1016/S0022-2860(00)00623-2
M. Colilla, M. Manzano, M. Vallet-Ragí, Recent advances in ceramic implants as drug delivery systems for biomedical applications. Int. J. Nanomed. 3, 403–414 (2008). https://doi.org/10.2147/IJN.S3548
T. Nii, F. Ishii, Encapsulation efficiency of water-soluble and insoluble drugs in liposomes prepared by the microencapsulation vesicle method. Int. J. Pharm. 298, 198–205 (2005). https://doi.org/10.1016/j.ijpharm.2005.04.029
V. Mišković-Stanković, A. Janković, S. Eraković, K.Y. Rhee, Graphene based biomedical composite coatings produced by electrophoretic deposition on titanium. Eurasian Chem. J. 17, 3–15 (2015)
W.T. Al-Jamal, K. Kostarelos, Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res. 44, 1094–1104 (2011). https://doi.org/10.1021/ar200105p
K.P. Sanosh, M.C. Chu, A. Balakrishnan, T.N. Kim, S.J. Cho, Utilization of biowaste eggshells to synthesize nanocrystalline hydroxyapatite powders. Mater. Lett. 63, 2100–2102 (2009). https://doi.org/10.1016/j.matlet.2009.06.062
V. Hengst, C. Oussoren, T. Kissel, G. Storm, Bone targeting potential of bisphosphonate-targeted liposomes. Preparation, characterization and hydroxyapatite binding in vitro. Int. J. Pharm. 331, 224–227 (2007). https://doi.org/10.1016/j.ijpharm.2006.11.024
Y. Cai, T. Gao, S. Fu, P. Sun, Development of zoledronic acid functionalized hydroxyapatite loaded polymeric nanoparticles for the treatment of osteoporosis. Exp. Ther. Med. 16, 704–710 (2018). https://doi.org/10.3892/etm.2018.6263
Q. Xu, Y. Tanaka, J.T. Czernuszka, Encapsulation and release of a hydrophobic drug from hydroxyapatite coated liposomes. Biomaterials 28, 2687–2694 (2007). https://doi.org/10.1016/j.biomaterials.2007.02.007
M. Stigter, J. Bezemer, K. De Groot, P. Layrolle, Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J. Control Release 99, 127–137 (2004). https://doi.org/10.1016/j.jconrel.2004.06.011
K. Yamamura, H. Iwata, T. Yotsuyanagi, Synthesis of antibiotic-loaded hydroxyapatite beads and in vitro drug release testing. J. Biomed. Mater. Res. 26, 1053–1064 (1992). https://doi.org/10.1002/jbm.820260807
M. Danaei, M. Dehghankhold, S. Ataei, F. Hasanzadeh Davarani, R. Javanmard, A. Dokhani, S. Khorasani, M.R. Mozafari, Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10, 1–17 (2018). https://doi.org/10.3390/pharmaceutics10020057
G. Pabst, N. Kučerka, M.-P. Nieh, J. Katsaras, Liposomes, Lipid Bilayers and Model Membranes: From Basic Research to Application, 1st edn. (CRC Press, Boca Raton, 2014). https://doi.org/10.1201/b16617
E. de Paula, J.D. Oliveira, F.F. de Lima, L.N. de Morais Ribeiro, Liposome-based delivery of therapeutic agents, in Controlled Drug Delivery Systems, 1st edn., ed. by E.C. Opara (Taylor & Francis, New York, 2020), pp. 299–324. https://doi.org/10.1201/9780429197833-16
L. Sercombe, T. Veerati, F. Moheimani, S.Y. Wu, A.K. Sood, S. Hua, Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 6, 1–13 (2015). https://doi.org/10.3389/fphar.2015.00286
K. Sarabandi, S.M. Jafari, M. Mohammadi, Z. Akbarbaglu, A. Pezeshki, M. KhakbazbHeshmati, Production of reconstitutable nanoliposomes loaded with flaxseed protein hydrolysates: stability and characterization. Food Hydrocoll. 96, 442–450 (2019). https://doi.org/10.1016/j.foodhyd.2019.05.047
C.F. de Freitas, I.R. Calori, A.C.P. da Silva, L.V. de Castro, F. Sato, D. Silva Pellosi, A.L. Tessaro, W. Caetano, N. Hioka, PEG-coated vesicles from Pluronic/lipid mixtures for the carrying of photoactive erythrosine derivatives. Colloids Surf. B 175, 530–544 (2019). https://doi.org/10.1016/j.colsurfb.2018.12.031
H.T. Schmidt, A.E. Ostafin, Liposome directed growth of calcium phosphate nanoshells. Adv. Mater. 14, 532–535 (2002). https://doi.org/10.1002/1521-4095(20020404)14:7%3c532::AID-ADMA532%3e3.0.CO;2-4
M.G. Bellino, A.E. Regazzoni, Coating liposomes with yttrium basic carbonate: making hybrid nanocapsules. J. Colloid Interface Sci. 333, 812–815 (2009). https://doi.org/10.1016/j.jcis.2009.02.035
A. Fihri, C. Len, R.S. Varma, A. Solhy, Hydroxyapatite: a review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 347, 48–76 (2017). https://doi.org/10.1016/j.ccr.2017.06.009
Q. Wang, Y. Chao, Multifunctional quantum dots and liposome complexes in drug delivery. J. Biomed. Res. 32, 91–106 (2017). https://doi.org/10.7555/JBR.31.20160146
J. Batalla, H. Cabrera, E. San Martín-Martínez, D. Korte, A. Calderón, E. Marín, Encapsulation efficiency of CdSe/ZnS quantum dots by liposomes determined by thermal lens microscopy. Biomed. Opt. Express 6, 3898 (2015). https://doi.org/10.1364/BOE.6.003898
N.F. Mohammad, R. Othman, F. Yee-Yeoh, Nanoporous hydroxyapatite preparation method for drug delivery. Rev. Adv. Mater. Sci. 38, 138–147 (2014)
E. de Paula, S. Schreier, Use of a novel method for determination of partition coefficients to compare the effect of local anesthetics on membrane structure. Biochim. Biophys. Acta. 1240, 25–33 (1995). https://doi.org/10.1016/0005-2736(95)00155-6
L.F. Fraceto, A. Spisni, S. Schreier, E. De Paula, Differential effects of uncharged aminoamide local anesthetics on phospholipid bilayers, as monitored by 1H-NMR measurements. Biophys. Chem. 115, 11–18 (2005). https://doi.org/10.1016/j.bpc.2004.12.003
H. Gheisari, E. Karamian, M. Abdellahi, A novel hydroxyapatite–Hardystonite nanocomposite ceramic A novel hydroxyapatite–Hardystonite nanocomposite ceramic. Ceram. Int. 41, 5967–5975 (2015). https://doi.org/10.1016/j.ceramint.2015.01.033
H.T. Schmidt, B.L. Gray, P.A. Wingert, A.E. Ostafin, Assembly of aqueous-cored calcium phosphate nanoparticles for drug delivery. Chem. Mater. 16, 4942–4947 (2004). https://doi.org/10.1021/cm040056i
M.B. Abramson, W.T. Norton, R. Katzman, Study of ionic structures in phospholipids by infrared spectra. J. Biol. Chem. 240, 2389–2395 (1965)
D.A. Skoog, S.R. Crouch, F.J. Holler, Instrumental Analysis Principles, 7th edn. (Cengage Learning, Boston, 2006)
K. Tahara, S. Fujimoto, F. Fujii, Y. Tozuka, T. Jin, H. Takeuchi, Quantum dot-loaded liposomes to evaluate the behavior of drug carriers after oral administration. J. Pharm. 2013, 1–6 (2013). https://doi.org/10.1155/2013/848275
L.W. Zhang, C.J. Wen, S.A. Al-Suwayeh, T.C. Yen, J.Y. Fang, Cisplatin and quantum dots encapsulated in liposomes as multifunctional nanocarriers for theranostic use in brain and skin. J. Nanoparticle Res. (2012). https://doi.org/10.1007/s11051-012-0882-9
S.R. Schaffazick, A.R. Pohlmann, T. Dalla-Costa, S.S. Guterres, Freeze-drying polymeric colloidal suspensions: nanocapsules, nanospheres and nanodispersion. A comparative study. Eur. J. Pharm. Biopharm. 56, 501–505 (2003). https://doi.org/10.1016/S0939-6411(03)00139-5
S.A. Lourenço, et al., Surface Engineering in Alloyed CdSe/CdSexCdS1–x/CdS Core-Shell Colloidal Quantum Dots for Enhanced Optoelectronic Applications, in Emerging Research in Science and Engineering Based on Advanced Experimental and Computational Strategies. Engineering Materials, ed. by F. La Porta, C. Taft (Springer, Cham, 2020). https://doi.org/10.1007/978-3-030-31403-3_7
H. Jin, B. Baek, D. Kim, F. Wu, J.D. Batteas, J. Cheon, D.H. Son, Effects of direct solvent-quantum dot interaction on the optical properties of colloidal monolayer WS2 quantum dots. Nano Lett. 17, 7471–7477 (2017). https://doi.org/10.1021/acs.nanolett.7b03381
Q. Xu, J.T. Czernuszka, Controlled release of amoxicillin from hydroxyapatite-coated poly(lactic-co-glycolic acid) microspheres. J. Control Release 127, 146–153 (2008). https://doi.org/10.1016/j.jconrel.2008.01.017
A.R. de Araújo, L.N.M. de Ribeiro, E. de Paula, Lipid-based carriers for the delivery of local anesthetics. Expert Opin. Drug Deliv. 16, 701–714 (2019). https://doi.org/10.1080/17425247.2019.1629415
H.P. Thakkar, A.K. Baser, M.P. Parmar, K.H. Patel, R. Ramachandra Murthy, Vincristine-sulphateloaded liposome-templated calcium phosphate nanoshell as potential tumor-targeting delivery system. J. Liposome Res. 22, 139–147 (2012). https://doi.org/10.3109/08982104.2011.633266
C.D.M. Donegá, P. Liljeroth, D. Vanmaekelbergh, Physicochemical evaluation of the hot-injection method, a synthesis route for monodisperse nanocrystals. Small 1, 1152–1162 (2005). https://doi.org/10.1002/smll.200500239
S. Hua, S.Y. Wu, The use of lipid-based nanocarriers for targeted pain therapies. Front. Pharmacol. 4, 143–150 (2013). https://doi.org/10.3389/fphar.2013.00143
D. Zucker, D. Marcus, Y. Barenholz, A. Goldblum, Liposome drugs’ loading efficiency: a working model based on loading conditions and drug’s physicochemical properties. J. Control. Release. 139, 73–80 (2009). https://doi.org/10.1016/j.jconrel.2009.05.036
Acknowledgments
The authors are thankful to the Spectroscopy laboratory at the State University of Londrina (UEL), and Labmult—Laboratory of Technological Federal University of Paraná, Londrina, PR, for use of their facilities. The capes-ds scholarship program (Grant Number 1795788).
Funding
This study was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes-ds program) (grant number 1795788).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lopes, F.F., de Freitas, C.F., de Paula, E. et al. Hydroxyapatite-coated liposomes for the controlled release of quantum dots and bupivacaine. Journal of Materials Research 36, 3021–3030 (2021). https://doi.org/10.1557/s43578-021-00292-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-021-00292-5