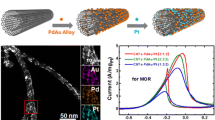
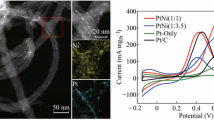
Abstract
This work reports the facile synthesis of Pt-Mn nanoparticles supported on multiwall carbon nanotubes using the Brust–Schiffrin method and their electrochemical performance during methanol electro-oxidation reaction. According to the topographical and structural results, the Pt-Mn nanoparticles present high dispersion with a narrow size distribution of ~ 2.5 nm. Meanwhile, the electrochemical evaluation exhibits a greatly enhanced electrocatalytic activity. The electrocatalyst's performance improves as Mn increased up to 40 at.% in Pt nanoparticles, but it decreases as Mn reaches 50 at.%. The electrocatalytic activity fall could be attributed to a reduction on active sites favorable to the dissociation and dehydrogenation of methanol. Density functional theory computations reveal that the partial density of states (PDOS) associated with [Mn]3d orbitals could be directly correlated with the observed electrocatalytic behavior. The respective maximum PDOS contribution at the Fermi level also corresponds to the most active electrocatalyst, which contains Mn of ~ 40 at.%.
Graphic abstract







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
S.S. Munjewar, B.T. Shashikant, K. Mallick Ranjan, Approaches to overcome the barrier issues of passive direct methanol fuel cell. Renew. Sustain. Energy Rev. 67, 1087–1104 (2017). https://doi.org/10.1016/j.rser.2016.09.002
L.C. Ordóñez, B. Escobar, R. Barbosa, Y. Verde-Gómez, Enhanced performance of direct ethanol fuel cell using Pt/MWCNTs as anodic electrocatalyst. J. Appl. Electrochem. 45, 1205–1210 (2015). https://doi.org/10.1007/s10800-015-0854-8
L. Gong, Z. Yang, K. Li, W. Xing, Ch. Liu, J. Ge, Recent development of methanol electro-oxidation catalysts for direct methanol fuel cell. J. Energy Chem. 7, 1618–1628 (2018). https://doi.org/10.1016/j.jechem.2018.01.029
J. Oh, H. Choi, M. Kim, Y. Kim, C. Park, Effects of morphological characteristics of Pt nanoparticles supported on poly(acrylic acid)-wrapped multiwalled carbon nanotubes on electrochemical performance of direct methanol fuel cells. J. Mater. Res. 27(15), 2035–2045 (2012). https://doi.org/10.1557/jmr.2012.156
M.J. Lee, J.S. Kang, Y.S. Kang, D.Y. Chung, H. Shin, C.Y. Ahn, S. Park, M.J. Kim, S. Kim, K.S. Lee, Y.E. Sung, Understanding the bifunctional effect for removal of co poisoning: blend of a platinum nanocatalyst and hydrous ruthenium oxide as a model system. Acs Catal. 6(4), 2398–407 (2016)
D. Cao, G.Q. Lu, A. Wieckowski, S.A. Wasileski, M. Neurock, Mechanisms of methanol decomposition on Pt: a combined experimental and ab initio approach. J. Phys. Chem. B 109, 11622–11533 (2005). https://doi.org/10.1021/jp0501188
L. Li, Y. Xing, Pt-Ru nanoparticles supported on carbon nanotubes as fuel cell catalysts. J. Phys. Chem. C 111, 2803–2808 (2007). https://doi.org/10.1021/jp0655470
L. Yang, J. Ge, Ch. Liu, G. Wang, W. Xing, Approaches to improve the performance of anode methanol oxidation reaction-a short review. Curr. Opin. Electrochem. 4, 83–88 (2017). https://doi.org/10.1016/j.coelec.2017.10.018
J.H. Bae, J.-H. Park, M.-K. Seo, S. Kim, Synthesis and electrochemical performances of platinum decorated polydopamine-coated carbon nanotubes/graphene composites as fuel cell catalysts. J. Alloy Compd. 822, 153586 (2020). https://doi.org/10.1016/j.jallcom.2019.153586
Y. Huang, J. Cai, Y. Guo, Roles of Pb and MnOx in PtPb/MnOx-CNTs catalyst for methanol electro-oxidation. Int. J. Hydrogen Energy 37, 1263–1271 (2012). https://doi.org/10.1016/j.ijhydene.2011.10.002
H. Zheng, M.S. Matseke, T.S. Munonde, The unique Pd@Pt/C core-shell nanoparticles as methanol-tolerant catalysts using sonochemical synthesis. Ultrason. Sonochem. 57, 166–171 (2019). https://doi.org/10.1016/j.ultsonch.2019.05.023
D. Friebel, V. Viswanathan, D.J. Miller, T. Annivev, H. O’Gasawara, A.H. Larsen, C.P. Orady, J.K. Norskov, A. Nilsson, Balance of nanostructure and bimetallic interactions in Pt model fuel cell catalysts: in situ XAS and DFT study. J. Am. Chem. Soc. 134, 9664–9671 (2012). https://doi.org/10.1021/ja3003765
Z. Lin, L. Ji, O. Toprakci, W. Krause, X. Zhang, Electrospun carbon nanofiber-supported Pt–Pd alloy composites for oxygen reduction. J. Mater. Res. 25(7), 1329–1335 (2010). https://doi.org/10.1557/JMR.2010.0163
J. Chang, L. Feng, K. Jiang, H. Xue, W. Cai, Ch. Liu, W. Xing, Pt–CoP/C as an alternative PtRu/C catalyst for direct methanol fuel cells. J. Mater. Chem. A 4, 18607–18613 (2016). https://doi.org/10.1039/C6TA07896F
Y. Sun, Y. Zhou, Ch. Zhu, L. Hu, M. Han, A. Wang, H. Huang, Y. Liu, Z. Kang, A Pt–Co3O4–CD electrocatalyst with enhanced electrocatalytic performance and resistance to CO poisoning achieved by carbon dots and Co3O4 for direct methanol fuel cells. Nanoscale 17, 5467–5474 (2017). https://doi.org/10.1039/C7NR01727H
J. Li, S. You, M. Liu, P. Zhang, Y. Dai, Y. Yu, N. Ren, J. Zou, ZIF-8-derived carbon-thin-layer protected WC/W24O68 micro-sized rods with enriched oxygen vacancies as efficient Pt co-catalysts for methanol oxidation and oxygen reduction. Appl. Catal. B 265, 118574 (2020). https://doi.org/10.1016/j.apcatb.2019.118574
N. Yu, L. Kuai, Q. Wang, B. Geng, Pt nanoparticles residing in the pores of porous LaNiO3 nanocubes as high-efficiency electrocatalyst for direct methanol fuel cell. Nanoscale 4, 5386–5393 (2012). https://doi.org/10.1039/C2NR31055D
Y. Kang, C.B. Murray, Synthesis and electrocatalytic properties of cubic Mn–Pt nanocrystals (nanocubes). J. Am. Chem. Soc. 132, 7568–7569 (2010). https://doi.org/10.1021/ja100705j
T.H.M. Housmans, A.H. Wonders, M.T. Koper, Structure sensitivity of methanol electro-oxidation pathways on platinum: an on-line electrochemical mass spectrometry study. J. Phys. Chem. B 110, 10021–10031 (2006). https://doi.org/10.1021/jp055949s
M.T.M. Koper, Structure sensitivity and nanoscale effects in electrocatalysis. Nanoscale 3, 2054–2073 (2011). https://doi.org/10.1039/C0NR00857E
J.S. Gullón, F.J.V. Iglesias, J.M. Feliu, Shape dependent electrocatalysis. Annu. Rep. Prog. Chem. C 107, 263–297 (2001). https://doi.org/10.1039/C1PC90010B
A. Demortière, P. Launois, N. Goubet, P.A. Albouy, C. Petit, Shape-controlled nanocubes and their assembly into two-dimensional and three-dimensional superlattices. J Phys Chem B. 112, 14583–14592 (2008). https://doi.org/10.1021/jp802081n
A. Demortière, C. Petit, First synthesis by liquid–liquid phase transfer of magnetic CoxPt100-x nanoalloys. Langmuir 23, 8575–8584 (2007). https://doi.org/10.1021/la700719h
S. Iijima, Helical microtubules of graphitic carbon. Nature 354, 56–58 (1991). https://doi.org/10.1038/354056a0
M.J.-F. Guinel, N. Brodusch, Y. Verde Gómez, B. Escobar Morales, R. Gauvin, Multiwalled carbon nanotubes decorated by platinum catalyst nanoparticles—examination and microanalysis using scanning and transmission electron microscopies. J. Microsc. 252, 49–57 (2013). https://doi.org/10.1111/jmi.12067
E.C. Landis, K.L. Klein, A. Liao, E. Pop, D.K. Hensley, A.V. Melechko, R.J. Hamers, Covalent functionalization and electron–transfer properties of vertically aligned carbon nanofibers: the importance of edge-plane sites. Chem. Mater. 22, 2357–2366 (2010). https://doi.org/10.1021/cm9036132
PCPDFWIN No. 04–0802, JCPDS–ICCD 2003.
PCPDFWIN No. 08–0415, JCPDS–ICCD 2003.
PCPDFWIN No. 04–0326, 04–0732, 12–0141, 24–0508, JCPDS–ICCD 2003
PCPDFWIN No. 17–0910, 32–0637, JCPDS–ICCD 2003.
D.H. Jung, S.J. Bae, S.J. Kim, K.S. Nahm, P. Kim, Effect of the Pt precursor on the morphology and catalytic performance of Pt-impregnated on Pd/C for the oxygen reduction reaction in polymer electrolyte fuel cell. Int J Hydrogen Energy 36, 9115–9192 (2011). https://doi.org/10.1016/j.ijhydene.2011.04.166
A. Khorsand, W.H.A. Majid, M.E. Abrishami, R. Parvizi, Synthesis, magnetic properties and X–ray analysis of Zn0.97X0.03O nanoparticles (X = Mn, Ni and Co) using Scherrer and size strain plot method. Solid State Sci. 14, 488–494 (2012). https://doi.org/10.1016/j.solidstatesciences.2012.01.019
G. Alonso-Núñez, L. Morales de la Garza, E. Rogel-Hernandez, E. Reynoso, A. Licea-Claverie, R.M. Felix-Navarro, G. Berhault, F. Paraguay-Delgado, New organometallic salts as precursors for the functionalization of carbon nanotubes with metallic nanoparticles. J. Nanoparticle Res. 13, 3643–3656 (2011). https://doi.org/10.1007/s11051-011-0283-5
P. Urchaga, S. Baranton, C. Coutanceau, G. Jerkiewicz, Evidence of an eley-rideal mechanism in the stripping of a saturation layer of chemisorbed CO on platinum nanoparticles. Langmuir 28, 13094–13104 (2011). https://doi.org/10.1021/la302388p
L. Liang, A. Hsu, D. Chu, R. Chen, Ethanol electro-oxidation on Pt/C and PtSn/C catalysts in alkaline and acid solutions. Int. J. Hydrogen Energy 35, 365–372 (2010). https://doi.org/10.1016/j.ijhydene.2009.10.058
J.S. Gullón, F.J.V. Iglesias, A.L. Cudero, E. Garnier, J.M. Feliu, A. Aldaz, Shape-dependent electrocatalysis: methanol and formic acid electro oxidation on preferentially oriented Pt nanoparticles. Phys. Chem. Chem. Phys. 10, 3689–3698 (2008). https://doi.org/10.1039/b802703j
C.L. Green, A. Kucernak, Determination of the platinum and ruthenium surface areas in platinum-ruthenium catalysts by underpotential deposition of copper 2: effect of surface composition on activity. J. Phys. Chem. B 106, 11446–11456 (2002). https://doi.org/10.1021/jp020859y
J. Suntivich, Z. Xu, C.E. Carlton, J. Kim, B. Han, S.W. Lee, N. Bonnet, N. Marzari, L.F. Allard, H.A. Gasteiger, K.H. Schifferli, Y.S. Horn, Surface composition tuning of Au–Pt bimetallic nanoparticles for enhanced carbon monoxide and methanol electro–oxidation. J. Am. Chem. Soc. 135, 7985–7991 (2013). https://doi.org/10.1021/ja402072r
J. Kang, S. Nam, Y. Oh, H. Choi, S. Wi, B. Lee, T. Hwang, S. Hong, B. Park, Electronic effect in methanol dehydrogenation on Pt surfaces: potential control during methanol electro oxidation. J Phys Chem Lett. 4, 2931–2936 (2013). https://doi.org/10.1021/jz401450v
A.M. Valenzuela-Muñiz, Y. Verde, M. Miki-Yoshida, G. Alonso-Núñez, Synthesis of multi-walled carbon nanotubes by spray-pyrolysis usinga new iron organometallic complex as catalytic agent. J. Nanosci. Nanotechnol. 8, 6456–6450 (2008). https://doi.org/10.1166/jnn.2008.18406
J.R. Rodríguez, R.M. Félix, E.A. Reynoso, Y.G. Ponce, Y.V. Gómez, S. Fuentes, G. Alonso, Synthesis of Pt and Pt–Fe nanoparticles supported on MWCNTs used as electrocatalysts in the methanol oxidation reaction. J Energy Chem. 23, 483–490 (2014). https://doi.org/10.1016/S2095-4956(14)60175-3
T. Ghosh, B.M. Leonard, Q. Zhou, F.J. DiSalvo, Pt alloy and intermetallic phases with V, Cr, Mn, Ni and Cu: synthesis as nanomaterials and possible application as fuel cell catalysts. Chem. Mater. 22, 2190–2202 (2010). https://doi.org/10.1021/cm9018474
P. Kim, J.B. Joo, W. Kim, J. Kim, I.K. Song, J. Yi, NaBH4-assited ethylene glycol reduction for preparation of carbon-supported Pt catalyst for methanol electro-oxidation. J. Power Sources 160, 987–990 (2006). https://doi.org/10.1016/j.jpowsour.2006.02.050
X. Ma, L. Luo, L. Zhu, L. Yu, L. Sheng, K. An, Y. Ando, X. Zhao, Pt–Fe catalyst nanoparticles supported on single-wall carbon nanotubes: direct synthesis and electrochemical performance for methanol oxidation. J Power Sources. 241, 274–280 (2013). https://doi.org/10.1016/j.jpowsour.2013.04.104
Software from Accelrys Inc.: http://www.accelrys.com.
B. Delley, An all-electron numerical method for solving local density functional for polyatomic molecules. J Chem Phys. 92, 508–517 (1990). https://doi.org/10.1063/1.458452
B. Delley, From molecules to solids with the DMol3 approach. J Phys Chem. 113, 7756–7764 (2000). https://doi.org/10.1063/1.1316015
J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys Rev Lett. 77, 3865–3865 (1996). https://doi.org/10.1103/PhysRevLett.77.3865
B. Delley, A scattering theoretic approach to scalar relativistic corrections on bonding. Int J Quant Chem. 69, 423–433 (1998). https://doi.org/10.1002/(SICI)1097-461X(1998)69:3%3c423::AID-QUA19%3e3.0.CO;2-2
Acknowledgments
We thank to A. Eloisa, Fco. Ruiz, I. Gradilla, A. Tiznado, and J. Peralta from CNyN-UNAM for their technical support. We acknowledge CONACyT (under projects 254667, 117373, 274314) for its financial support.
Funding
This study was supported by Consejo Nacional de Ciencia y Tecnología (Grant Nos. 254667, 117373, 274314).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study and manuscript equally.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Rodríguez, J.R., Verde-Gómez, Y., Díaz de León, J.N. et al. Effect of Pt-Mn nanoparticles supported on CNT in methanol electro-oxidation reaction, experimental, and theoretical studies. Journal of Materials Research 36, 4216–4226 (2021). https://doi.org/10.1557/s43578-021-00275-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-021-00275-6